Method for preparing 4-n-butyl resorcinol by one-pot method
A technology of n-butyl resorcinol and resorcinol, which is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as environmental pollution and production safety reduction, and achieve atomic utilization High efficiency, easy operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
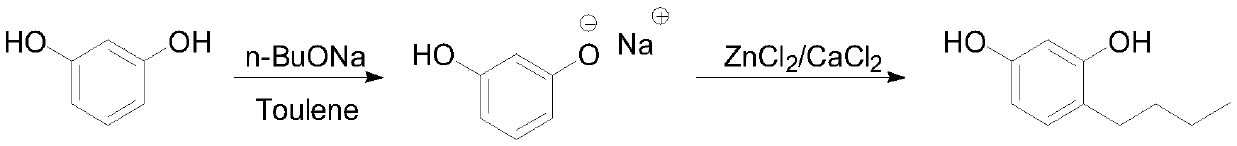
[0020]
[0021] Under the protection of nitrogen, put 110g (1mol) of resorcinol and 350g of toluene into the reaction bottle to mix and dissolve, then cool down to 10°C, add 98g (1.02mol) of sodium n-butoxide in batches, and control the temperature at 10-15°C, TLC (EA developer) After detecting that there are almost no remaining raw materials, add n-butanol (2.2mol), 27.2g (0.2mol) of zinc chloride and 1.1g of calcium chloride, raise the temperature to 40-45°C for 1 hour, and then raise the temperature to 102 ℃ to carry out reflux water separation, after 6 hours of water separation, sampling and quenching GC to detect resorcinol 1 HNMR (400MHz, CDCl 3 ):0.96(t,3H),1.35-1.62(m,4H),2.55(m,2H),6.15-6.24(m,2H),6.75(s,1H),8.46(s,1H),12.83( s, 1H).
Embodiment 2
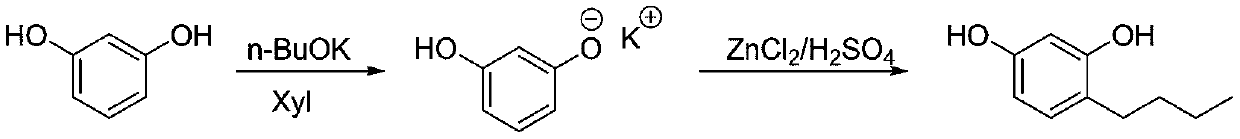
[0023]
[0024] Under the protection of nitrogen, put 110g (1mol) of resorcinol and 350g of xylene into the reaction bottle to mix and dissolve, then cool down to 10°C, add 113.3g (1.01mol) of potassium n-butoxide in batches, and control the temperature at 10-15°C , TLC (EA developer) detects that there is almost no remaining raw material, add n-butanol (2.2mol), zinc chloride 27.2g (0.2mol) and concentrated sulfuric acid 2.2g, heat up to 40-45°C for 1 hour, then heat up to Reflux at 112°C for water separation. After 4 hours of water separation, sample and quench GC to detect resorcinol 1 HNMR (400MHz, CDCl 3 ):0.96(t,3H),1.35-1.62(m,4H),2.55(m,2H),6.15-6.24(m,2H),6.75(s,1H),8.46(s,1H),12.83( s, 1H).
Embodiment 3
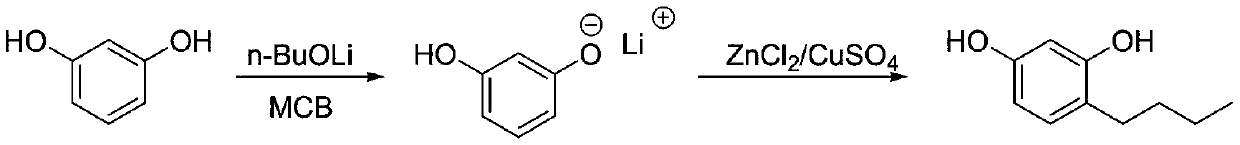
[0026]
[0027] Under the protection of nitrogen, put 110g (1mol) of resorcinol and 350g of m-chlorobenzene into the reaction bottle to mix and dissolve, then cool down to 10°C, add 81.7g (1.02mol) of lithium n-butoxide in batches, and control the temperature for 10-15 ℃, after TLC (EA developer) detects that almost no raw materials are left, add n-butanol (2.5mol), zinc chloride 272g (0.2mol) and anhydrous copper sulfate 1.5g, raise the temperature to 40-45℃ for 1 hour, and then Raise the temperature to 113°C for reflux and water separation. After 4 hours of water separation, take a sample and quench the GC to detect resorcinol <1%, cool down to room temperature, filter out the solid catalyst through diatomaceous earth, and add dilute sulfuric acid to adjust the pH=1 -2. Concentrate the organic phase to stagnant liquid, add water to replace, then add 660g water, 2.2g sodium thiosulfate and 5.5g activated carbon, heat up to reflux for 1 hour, heat filter to obtain a light ye...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com