Synthesis method of pregabalin intermediate 2-carboxyethyl-3-nitromethylene-5-methyl ethyl hexanoate
A technology of ethyl methylhexanoate and nitromethylene, which is applied in the field of synthesis of intermediates containing pregabalin, can solve the problems of high cost, many intermediates, long reaction time, etc. The effect of simple processing and low reaction cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 Synthesis of ethyl 2-carboxyethyl-5-methyl-2-hexanoate:
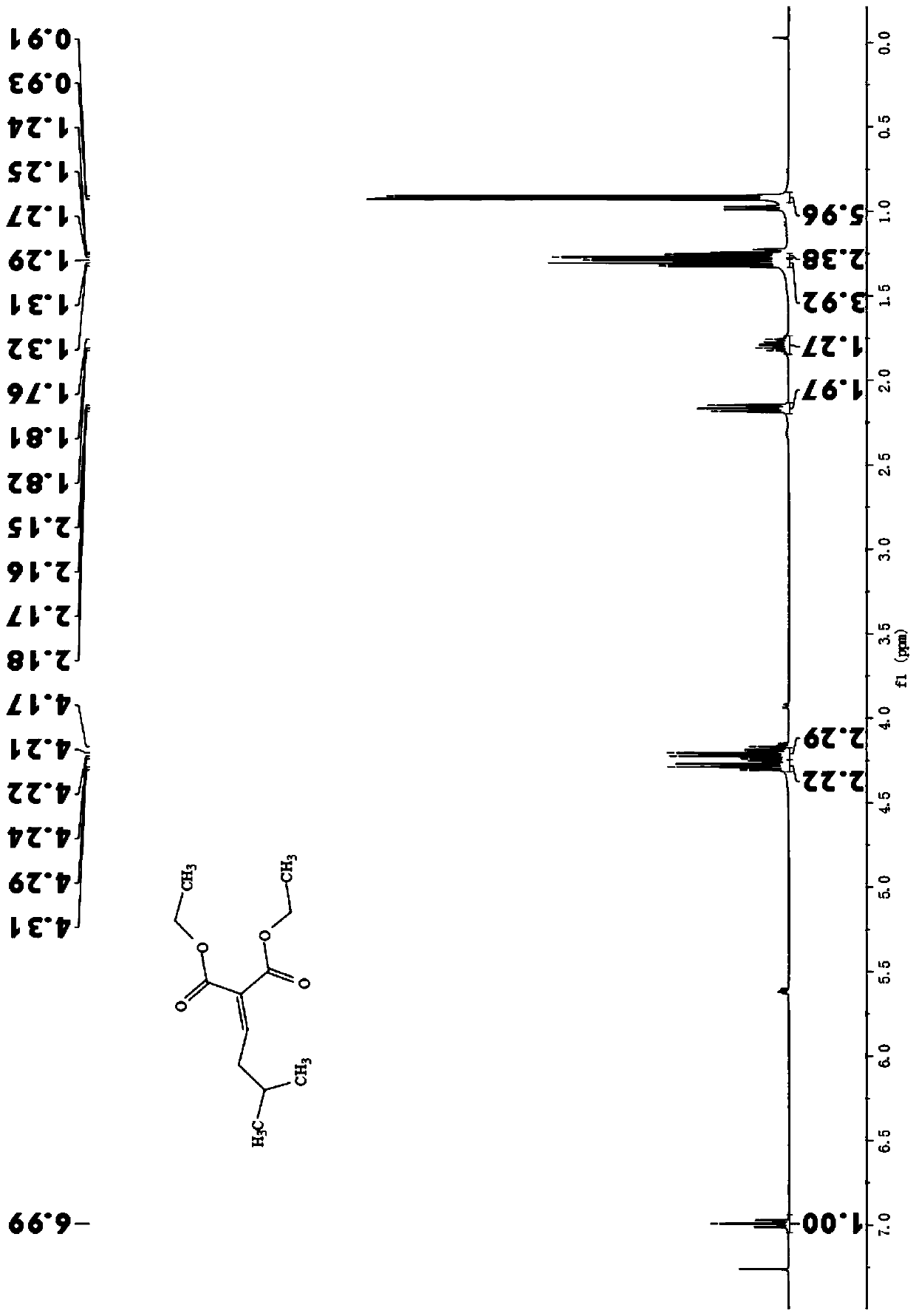
[0023] In a dry three-necked flask, add 1.4g (1mol) choline chloride and 1.2g (2mol) urea, stir and heat up to 80°C to obtain a colorless transparent liquid, which is the deep eutectic solvent: DES (chlorinated choline-urea); then add 0.5375g (1mol) isovaleraldehyde, 1.0g (1.0mol) diethyl malonate, stir and react at 80°C, monitor by TLC until the reaction is complete. After the reaction, the reaction mixture was cooled to room temperature, distilled water was added, extracted with ethyl acetate, and the organic phase was evaporated to remove the solvent to obtain ethyl 2-carboxyethyl-5-methyl-2-hexanoate, a colorless transparent liquid. Yield 96.6%. The water phase is evaporated to obtain DES, which can be recycled and reused.
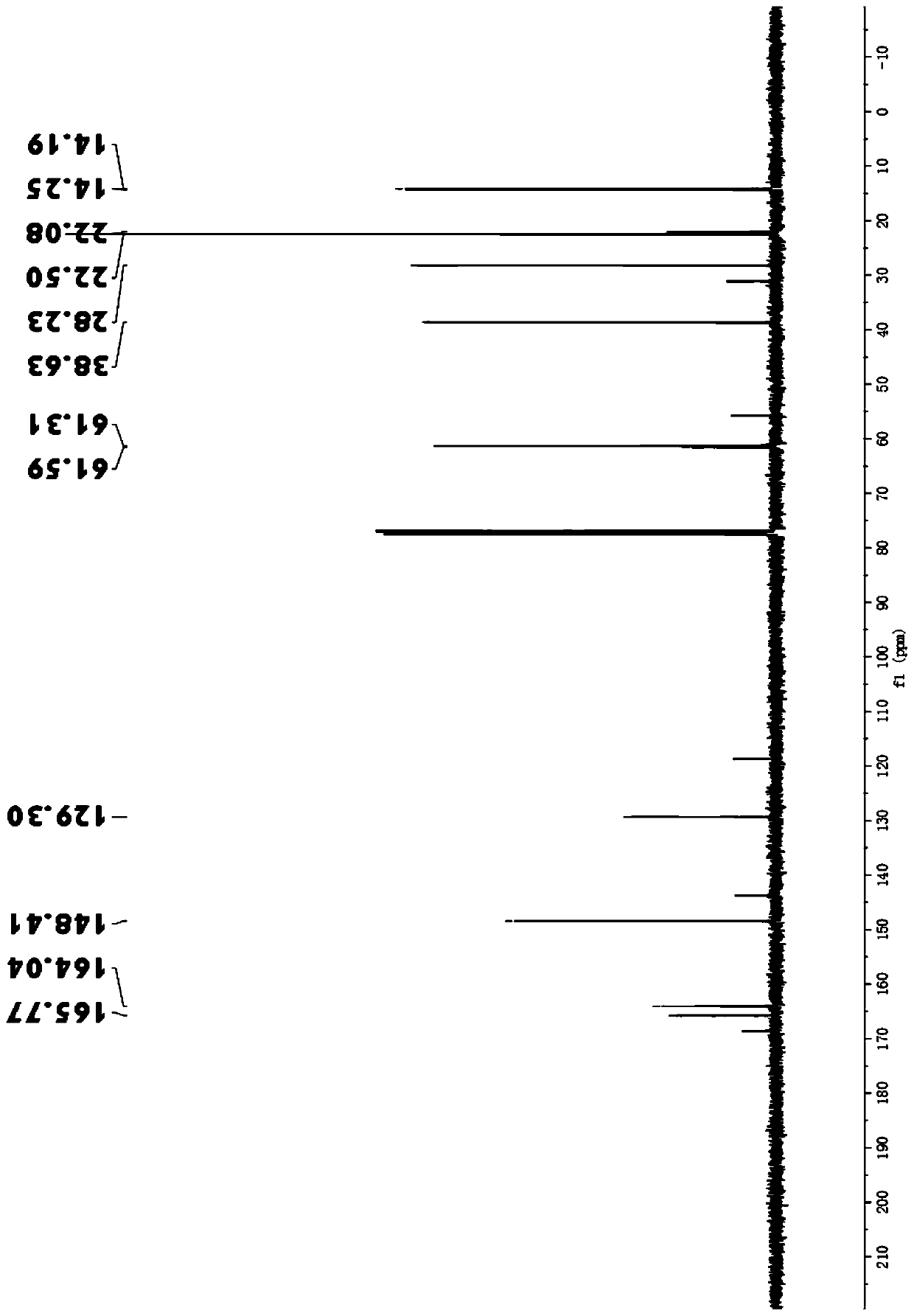
[0024] 1 H NMR (400MHz, CDCl 3 )δ:6.99(t,1H),4.17-4.31(m,4H),2.18-2.15(m,2H),1.76-1.82(m,1H),1.31-1.24(m,6H),0.91-0.93( m,6H).
[0025] 13 C NMR (101MHz, CDCl 3 )δ: 165....
Embodiment 2
[0026] Example 2 Synthesis of ethyl 2-carboxyethyl-3-nitromethylene-5-methylhexanoate:
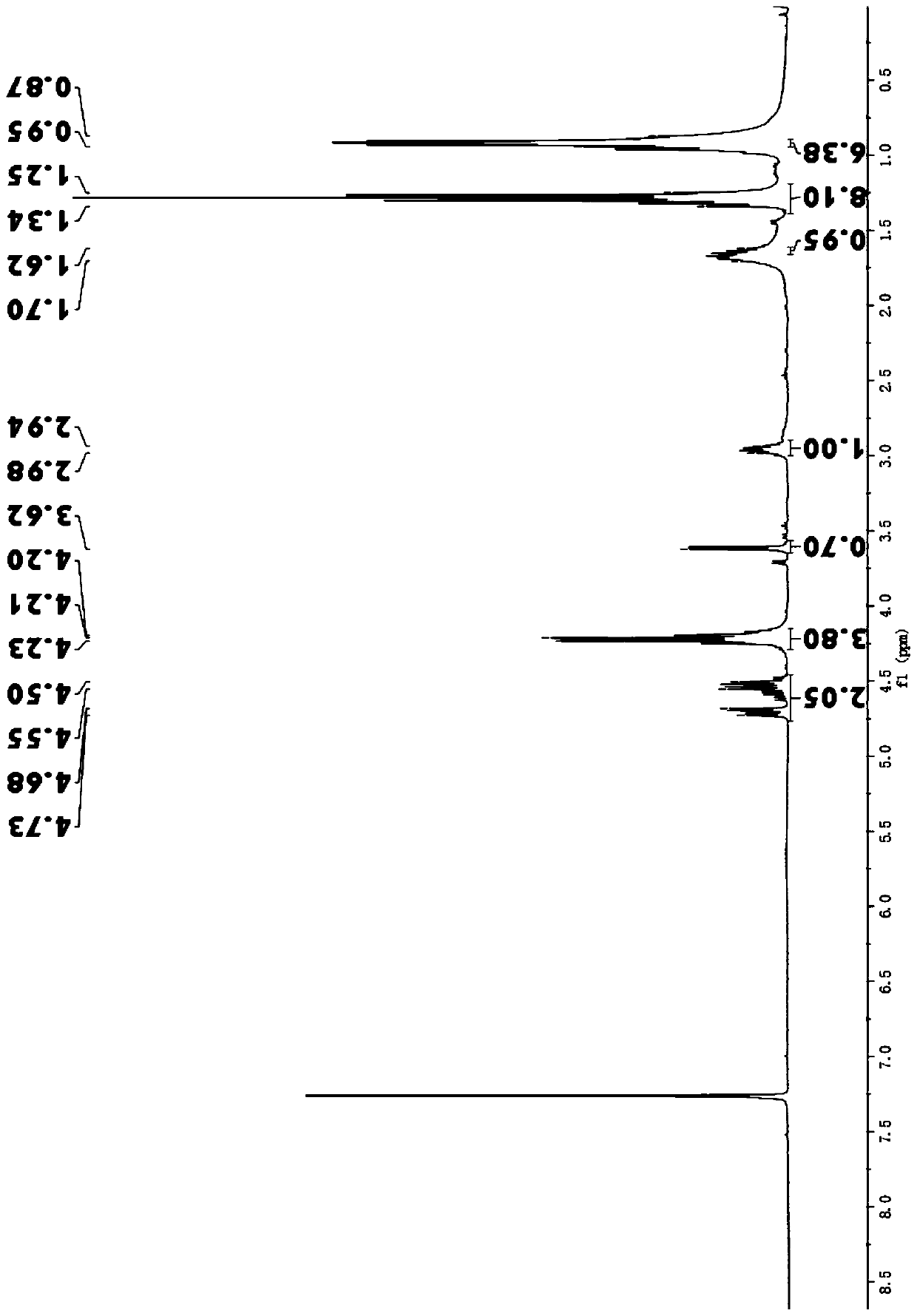
[0027] The second step: add 1.394g (1.0mol) 2-carboxyethyl-5-methyl-2-hexanoic acid ethyl ester, 0.447g (1.2mol) nitromethane, 0.293g (1.0mol) NaOH to the mortar , 1.01g (1.0mol) anhydrous K 2 CO 3 (Wherein E:F:G:H=1:1.2:1:1.1) Grinding reaction, TLC monitoring until the reaction is complete, add appropriate amount of distilled water, extract with ethyl acetate, evaporate the solvent from the organic layer to obtain 2-carboxyethyl-3 -Ethyl nitromethylene-5-methylhexanoate, yellow liquid, yield 96%.
[0028] 1 H NMR (400MHz, CDCl 3 )δ:4.73-4.50(q,2H),4.23-4.20(q,4H),3.62(d,2H),1.62-1.70(q,1H),1.25-1.34(d,8H),0.87-0.95( m, 6H).
Embodiment 3
[0029] Example 3 Synthesis of ethyl 2-carboxyethyl-3-nitromethylene-5-methylhexanoate:
[0030] Step 1: In a dry three-necked flask, add 1.4g (1mol) choline chloride and 1.2g (2mol) urea at room temperature, raise the temperature to 80°C, and stir magnetically until a colorless transparent liquid is obtained, that is, a deep eutectic solvent : DES (choline chloride-urea); then add 0.5375 (1 mol) isovaleraldehyde, 1.2 g (1.2 mol) diethyl malonate, heat up to 80 ° C, stir the reaction, TLC monitoring until the reaction is complete. After the reaction, cool to room temperature, pour into water, extract with ethyl acetate, evaporate the solvent from the organic layer to obtain ethyl 2-carboxyethyl-5-methyl-2-hexanoate, evaporate the water phase to recover DES, which can be reused . The second step: add 1.626g (1.0mol) ethyl 2-carboxyethyl-5-methyl-2-hexanoate, 0.521g (1.2mol) nitromethane, 0.284g (1mol) NaOH in the mortar, 0.980g (1mol) K 2 CO 3 , (wherein E:F:G:H=1:1.2:1:1.1)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com