Synthetic bioconjugates
A conjugate and biological technology, applied in the direction of non-active ingredient medical preparations, active ingredient-containing medical preparations, drug combinations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0253] Example 1. Synthesis of bioconjugates
[0254] Bioconjugates can be prepared according to the following scheme. Use an appropriate concentration of a chaotropic agent such as butanol, ethanol, guanidine chloride, lithium perchlorate, lithium acetate, magnesium chloride, phenol, propanol, sodium lauryl sulfate, thiourea, or urea (e.g. about 5M to about 10M urea), prepare a suitable reaction buffer (eg, 2-(N-morpholino)ethanesulfonic acid (MES)). The final pH was adjusted to pH about 4.5 to about 6 with 1 N HCl.
[0255] Hydrazide-functionalized peptides (eg, peptides 1-20 in Table 2) were dissolved in reaction buffer to 3 mg / mL. The peptide solution was prepared freshly prior to the coupling reaction. The corresponding biotinylated peptides were dissolved in reaction buffer to 3 mg / mL. Prepare the resulting biotinylated peptide solution freshly prior to the coupling reaction. Glycans (such as heparin (MW avg = 16 kDa)) was dissolved in reaction buffer to 20 mg / mL a...
Embodiment 2
[0265] Example 2. Collagen binding plate test
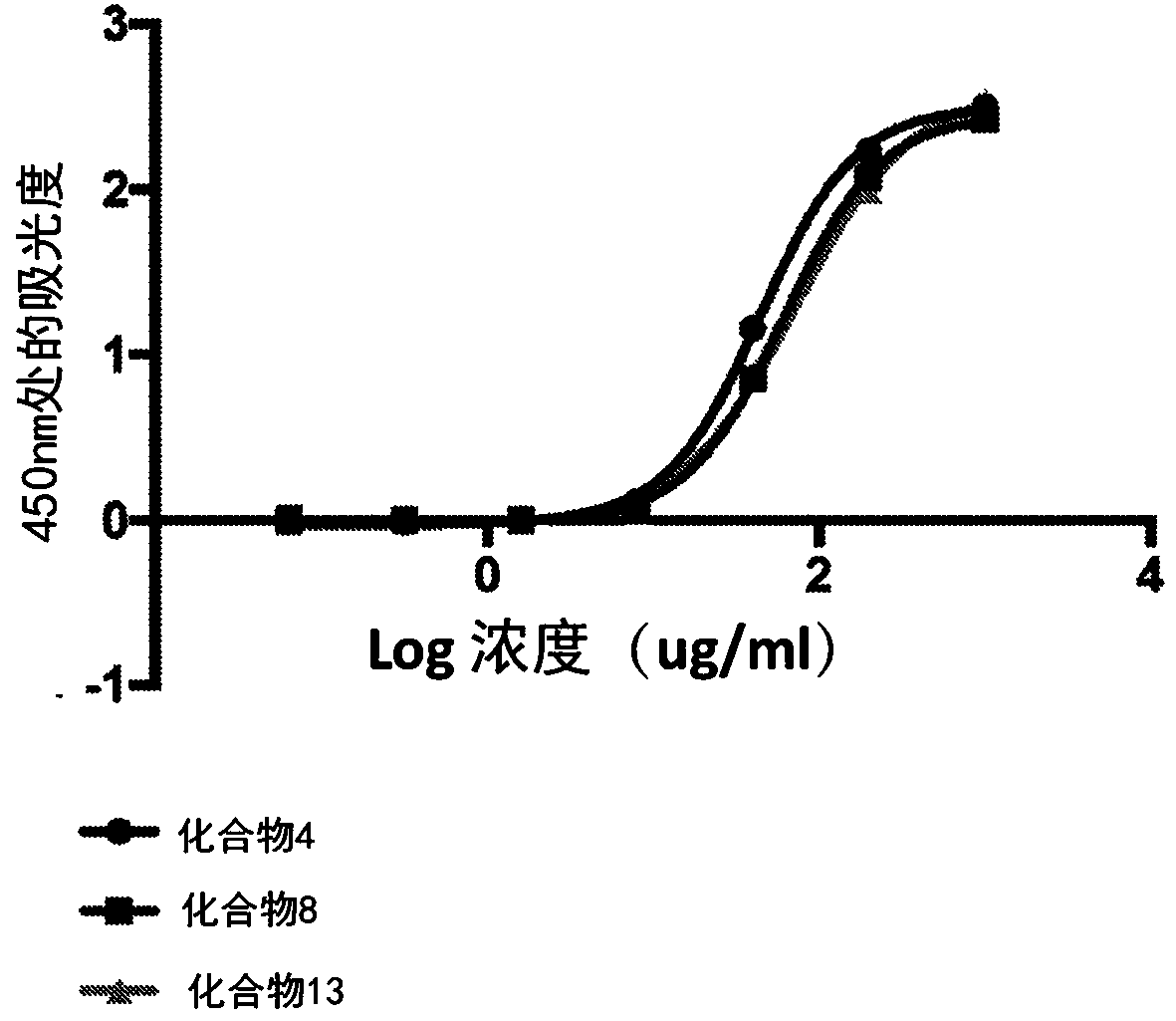
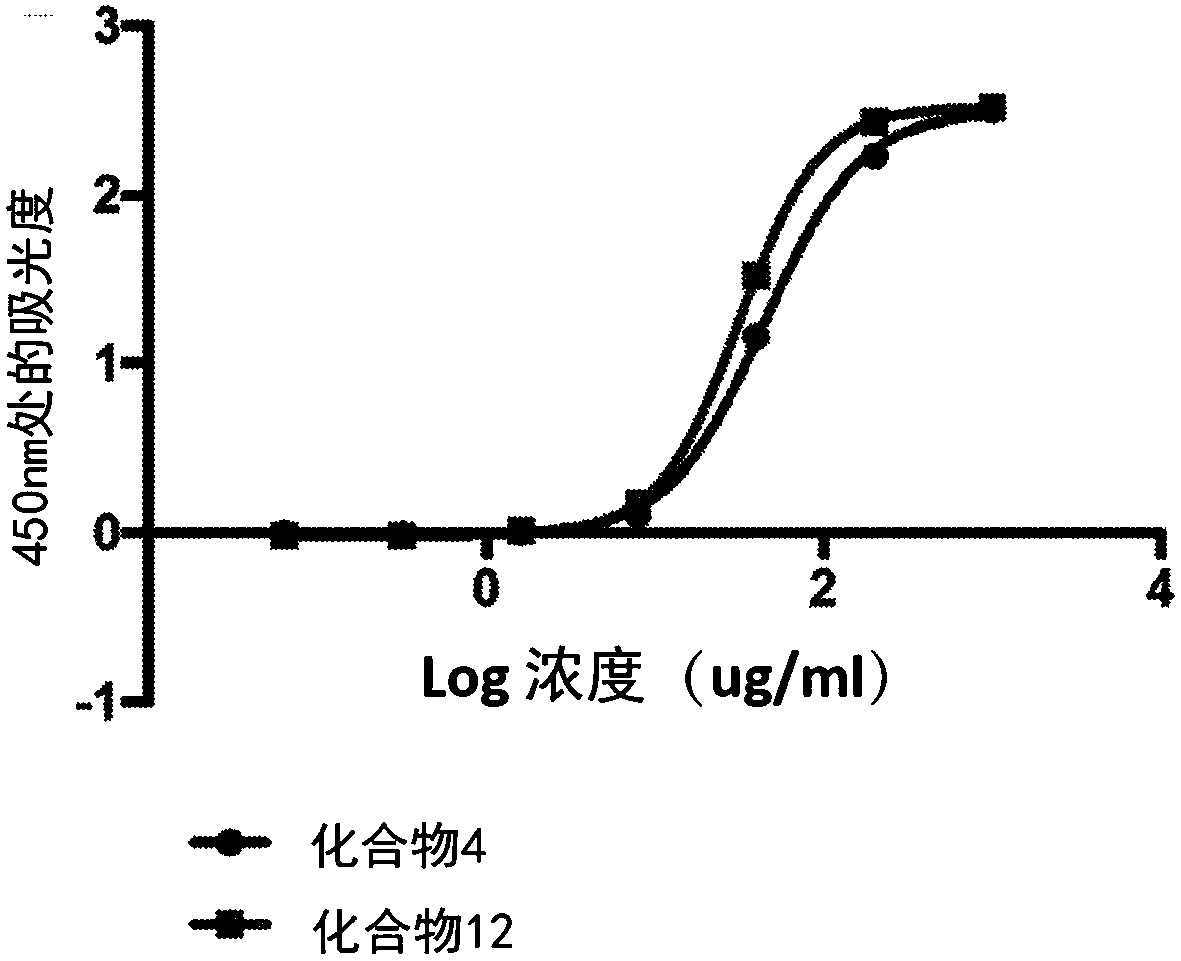
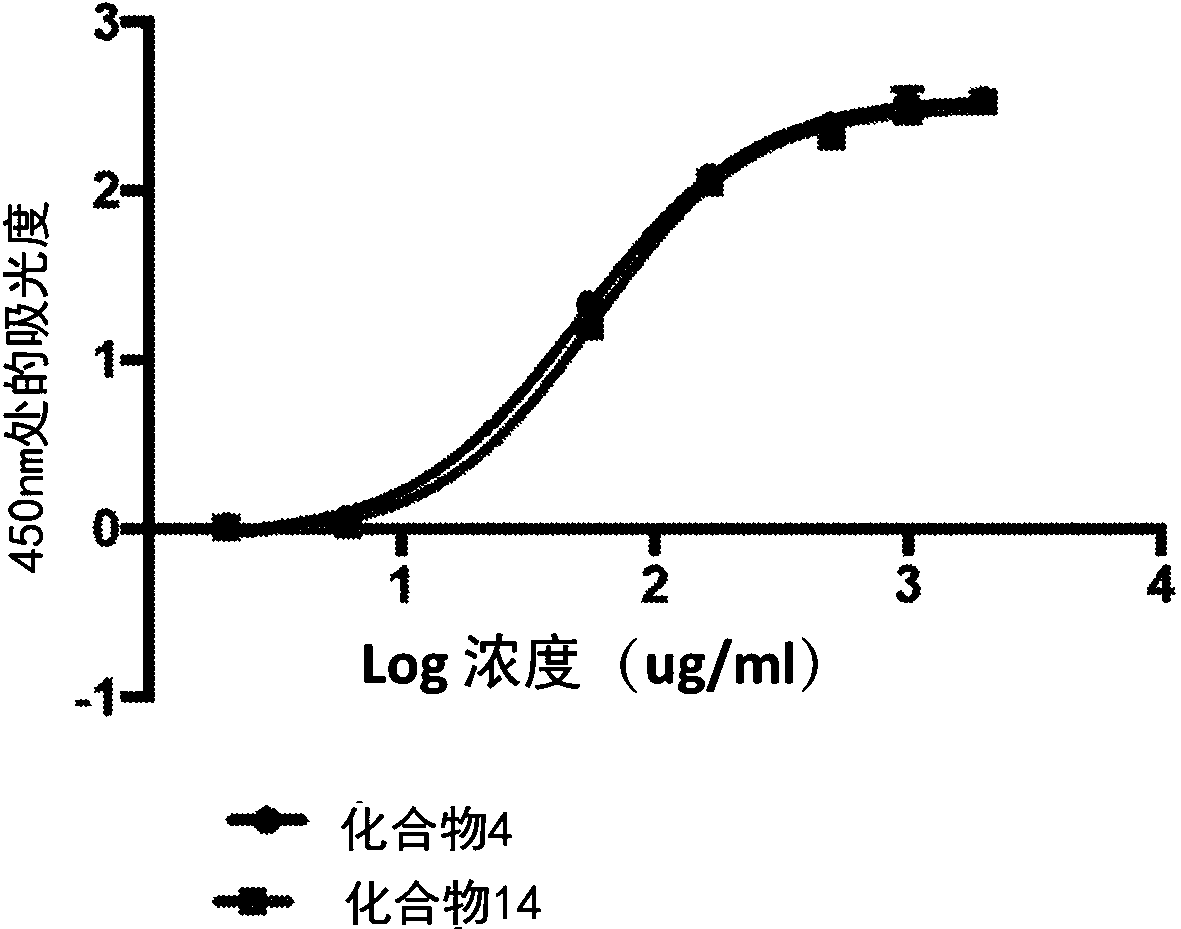
[0266] The following method was used to assess the binding affinity of the bioconjugates disclosed herein for collagen.
[0267] Collagen binding of the bioconjugate variants was compared by plate assay in which collagen was coated on 96-well plates. The collagen was coated on the high-binding plate with 50 μg / mL 0.02N acetic acid solution for 1 h at room temperature. Unbound collagen was washed with pH 7.4 IX PBS. Plates were then blocked in 1% milk in IX PBS for 1 hour at room temperature.
[0268] Bioconjugate variants containing biotinylated peptides were dissolved in 1% milk in 1X PBS pH 7.4 to a final concentration of 1 mg / mL. From this solution, 1OX serial dilutions were performed. Molecules were then incubated on blocked collagen-coated plates for 15 minutes at room temperature. Plates were then washed 3 times with 1X PBS containing 1% BSA and 0.2% Tween20.
[0269] Bound molecules were detected by streptavidin-HRP,...
Embodiment 3
[0282] Example 3. Platelet Aggregation
[0283] Type I fibrillar collagen was adsorbed onto Ibidi μ-slides by overnight incubation at 2–8 °C. Ibidi μ-slides were rinsed with phosphate buffered saline (PBS) and then blocked with 1% BSA in 1x PBS. 2mg / mL of compound 10 (such as Figure 12 B) and compound 1 (as Figure 12 Indicated in A) were applied to Ibidi μ-slides and allowed to incubate. After 1 hour, excess conjugate was washed away with 1x PBS. Freshly drawn human whole blood was prestained with Calcein AM (a live fluorescent cell marker). Blood was pumped through the channel using a syringe pump at a shear rate of 1000 s-1 for 10 min. Fluorescence microscopy was used to capture images of aggregated fluorescently labeled platelets as blood flowed across the Ibidi μ-slide. Figure 12 shows compound 10 (small Figure 12 B) Inhibition of platelet binding to type I fibrillar collagen.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com