In-vitro evaluation model and method for hemostatic performance of toothpaste containing bletilla striata extract
A technology for evaluating models and extracts, applied in the preparation of test samples, measurement of color/spectral characteristics, measurement devices, etc., can solve the problems that the content has not been compared and analyzed in detail, so as to reduce the cost of in vivo tests, reduce and use , The effect of improving the efficiency of research work
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 toothpaste hemostatic performance evaluation model in vitro
[0038] To construct an in vitro evaluation model for the hemostatic performance of toothpaste containing Bletilla striata extract, the specific method is as follows:
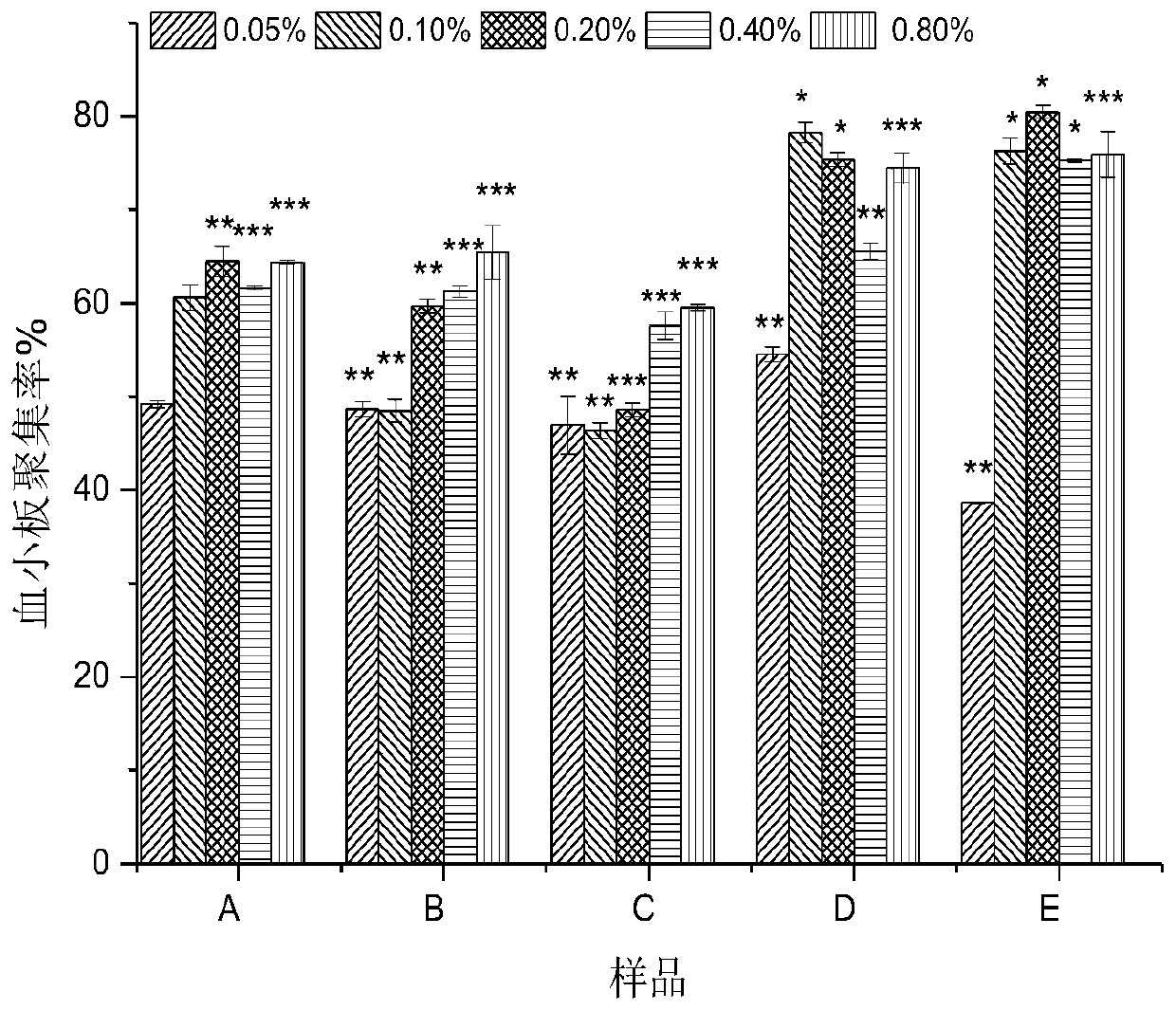
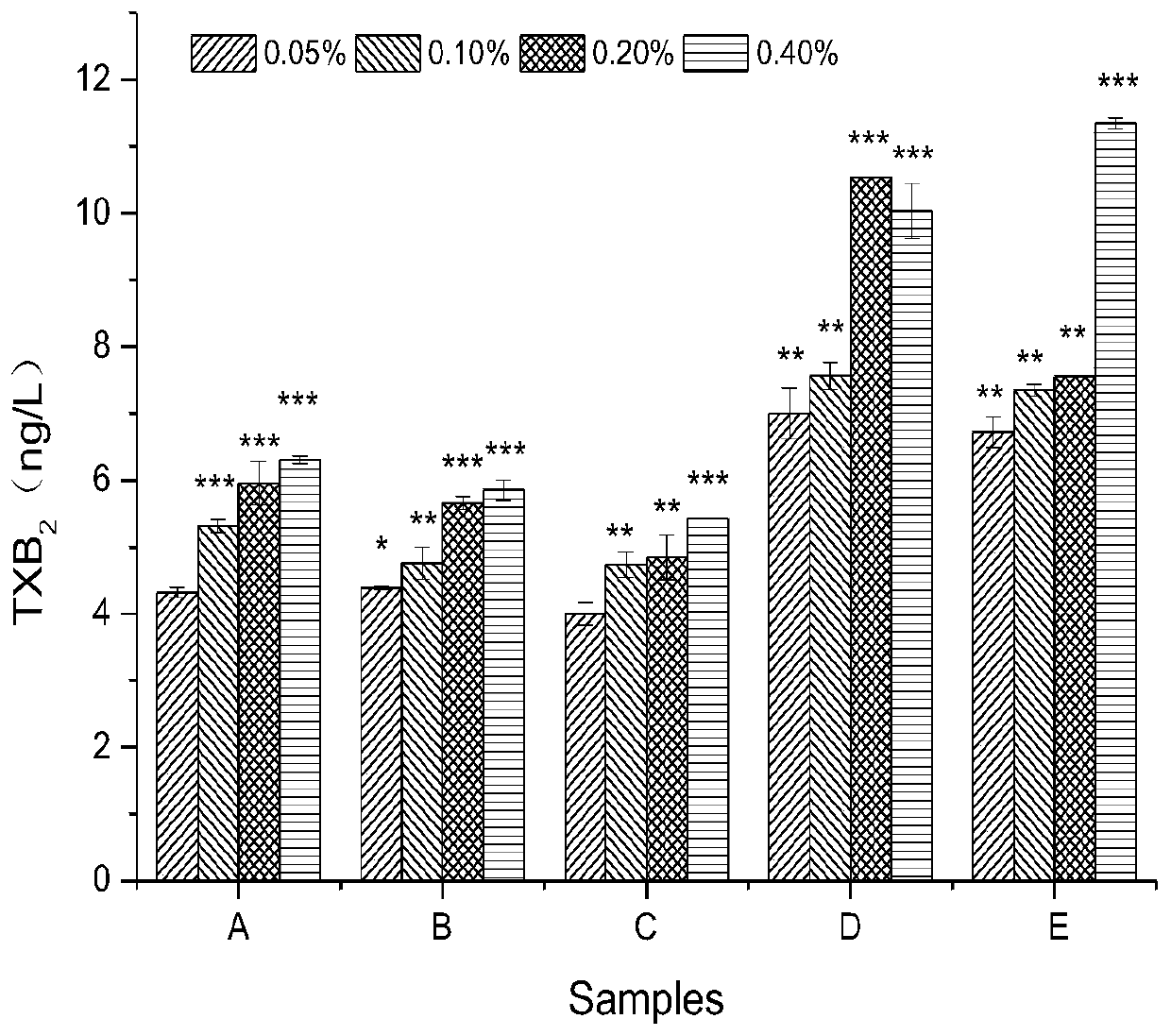
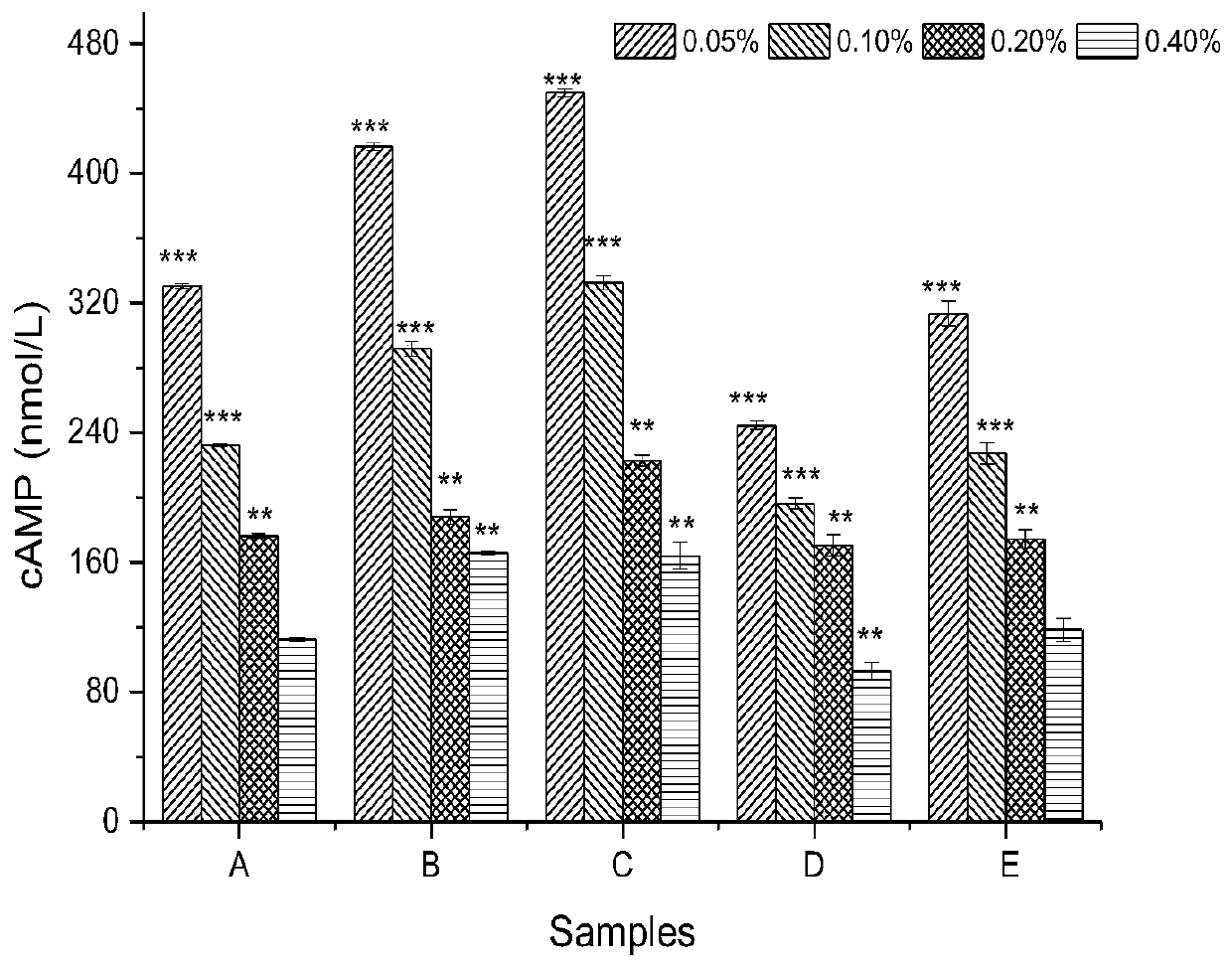
[0039] Collect arterial blood from female New Zealand white rabbits, centrifuge at 900rpm for 8min at 22°C, slowly transfer the supernatant liquid after centrifugation to obtain platelet-rich plasma; centrifuge the remaining part at 3000rpm for 10min, take the supernatant, and adjust the platelet density to 1×10 12 / L, to obtain platelet-poor plasma, put it in a 96-well plate, and detect the absorbance OD of the blank 0 , add different samples A to F groups (5uL / well), shake slightly. Then incubate the 96-well plate containing the samples at a constant temperature of 37°C for 5 minutes to fully react. Then add 5uL of ADP solution and shake slightly to induce platelet aggregation. After platelet aggregation occurs, the turbidity in t...
Embodiment 2
[0049] Embodiment 2 Animal experiments
[0050] Set toothpaste sample groups, respectively: A-Bletilla striata Ⅰ toothpaste group; B-Bletilla striata Ⅱ toothpaste group; C-negative toothpaste group; D-tranexamic acid toothpaste group; E-Bletilla striata extract group; F-control group ; G-blank group. IgA: F=311.7, a P<0.01, establish an animal gingival inflammation model, and verify the hemostatic performance evaluation model of toothpaste in vitro by observing the bleeding index (BOP) of the X-ray tissue and the content of immunoglobulins (IgA, IgM, IgG) in saliva instead of in vivo Feasibility of animal experiments.
[0051] IgA is secretory immunoglobulin A, which is the first immune defense line of the body against pathogens, effectively preventing harmful substances in the oral cavity from entering epithelial cells; IgM is immunoglobulin M, which is the earliest antibody in the immune response. Its content is used to evaluate the repairing effect of gingivitis in the o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com