A preparation method of high-efficiency recyclable lithium extraction membrane material for salt lake brine
A salt lake brine, high-efficiency technology, applied in chemical instruments and methods, water/sewage treatment, adsorption water/sewage treatment, etc., can solve the problems of unfavorable recycling, serious dissolution loss of lithium extraction, etc. The effect of fast desorption speed and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
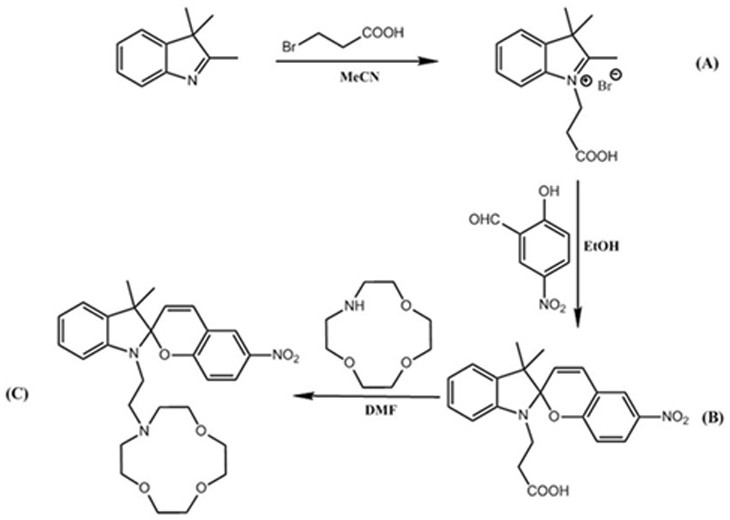
[0020] A method for preparing a high-efficiency recyclable membrane adsorption material suitable for high-magnesium-lithium ratio salt lake brine, the steps are as follows:
[0021] a. Dissolve 2,3,3-trimethylindole (3.18 g, 20 mmol) and 3-bromopropionic acid (6.08 g, 40 mmol) in 50 mL of acetonitrile, react at 85 °C for 22 h, and stop the reaction Afterwards, cool to room temperature, evaporate the solvent, wash the residue 3 times with ether, recrystallize with a mixed solution of dichloromethane: acetone = 1:5, filter with suction, and dry in vacuo to obtain product A;
[0022] b. Dissolve product A (3.11 g, 10 mmol) and 5-nitrosalicylaldehyde (1.85 g, 12 mmol) in 50 mL of absolute ethanol, drop into 7 mL of triethylamine, and react in the dark at 80 °C for 24 h, after the reaction stops, cool to room temperature, add deionized water, adjust the pH to acidic with dilute hydrochloric acid, filter with suction, and wash with deionized water for 3 times. Vacuum drying obtains...
Embodiment 2
[0026] (1) Configure Mg 2+ 、Na + 、K + , Li + The solutions with concentrations of 5 mg / L, 5 mg / L, 5 mg / L, and 1 mg / L were used as simulated brine for adsorption experiments, and the prepared membranes were subjected to adsorption experiments, and the maximum adsorption capacity was measured to be 28.7 mg / g, After 10 cycles, the adsorption capacity can still reach 98.3% of the maximum adsorption capacity.
[0027] (2) Configure Mg 2+ 、Na + 、K + , Li + The solutions with concentrations of 8 mg / L, 8 mg / L, 8 mg / L, and 1 mg / L were used as simulated brine for adsorption experiments, and the prepared membranes were subjected to adsorption experiments, and the maximum adsorption capacity was measured to be 26.3 mg / g, After 10 cycles, the adsorption capacity can still reach 96.1% of the maximum adsorption capacity.
[0028] (3) Configure Mg 2+ 、Na + 、K + , Li + The solutions with concentrations of 10 mg / L, 10 mg / L, 10 mg / L, and 1 mg / L were used as simulated brine for adsorp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com