Bistriazole ligand-regulated cadmium 5-nitroisophthalate complex, preparation method thereof, and application of complex as fluorescent probe
A technology of cadmium nitroisophthalate and bistriazole, which is applied in the direction of cadmium organic compounds, 2/12 organic compounds without C-metal bonds, fluorescence/phosphorescence, etc., to achieve good reproducibility and production High efficiency and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Dissolve 5-nitroisophthalic acid (21.1mg, 0.1mmol), sodium hydroxide (8.0mg, 0.2mmol), BTB (24.1mg, 0.1mmol) and cadmium nitrate (29.3mg, 0.1mmol) in water ( 10mL), sealed in a 16mL reaction kettle, heated to 160°C at a rate of 10°C per hour, maintained at this temperature for 3 days, and then naturally cooled to room temperature to obtain colorless blocky crystals, which were separated. After washing and drying successively, the target product was obtained with a yield of about 49%. The main infrared absorption peak is: 3336.85cm -1 , 3142.04cm -1 , 1624.06cm -1 , 1606.70cm -1 ,1556.55cm -1 ,1529.55cm -1 ,1354.03cm -1 , 1136.07cm -1 , 1024.35cm -1 ,889.18cm -1 ,786.94cm -1 , 734.88cm -1 .
[0042] Get the bisazole ligand that makes in Example 1 and regulate the cadmium complex of 5-nitroisophthalate for further characterization, and its process is as follows:
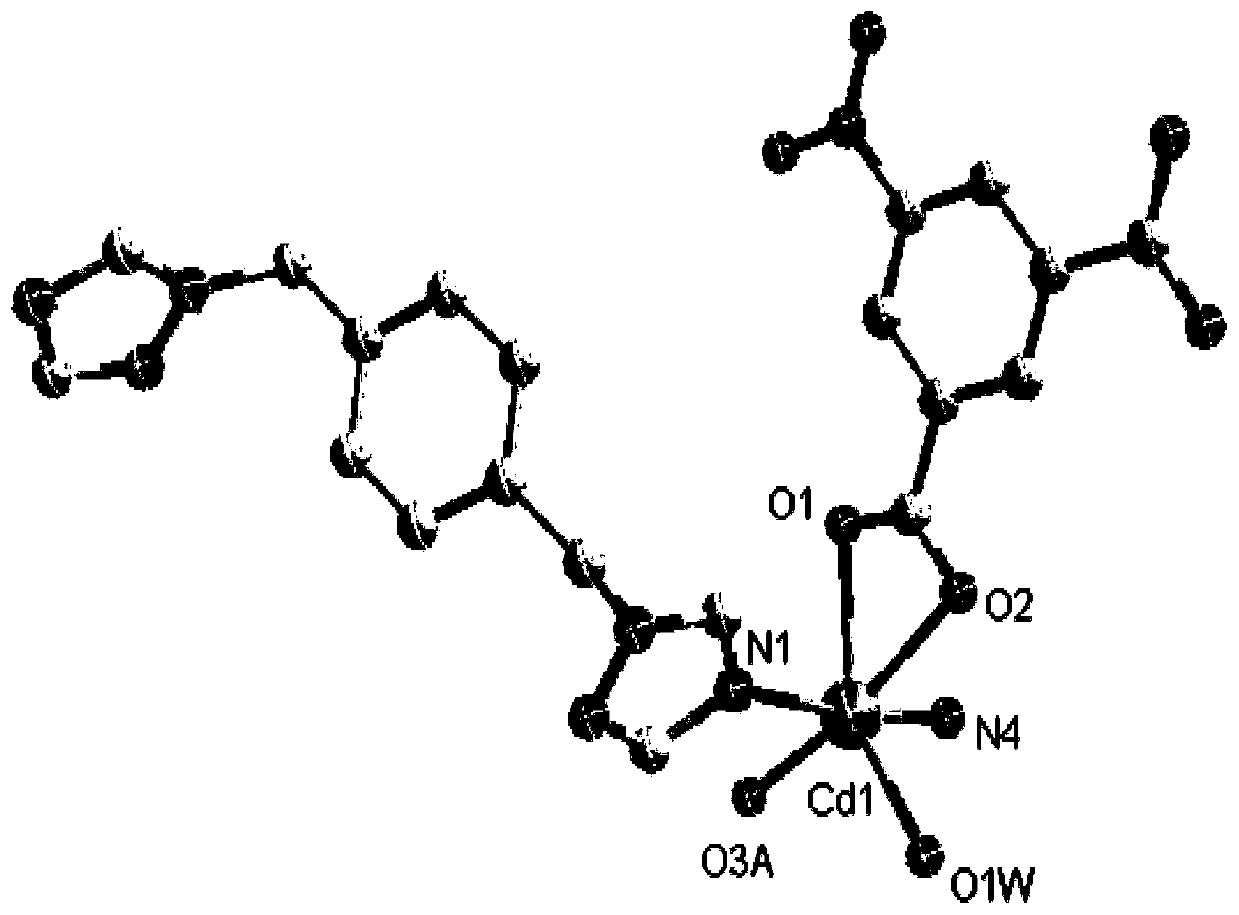
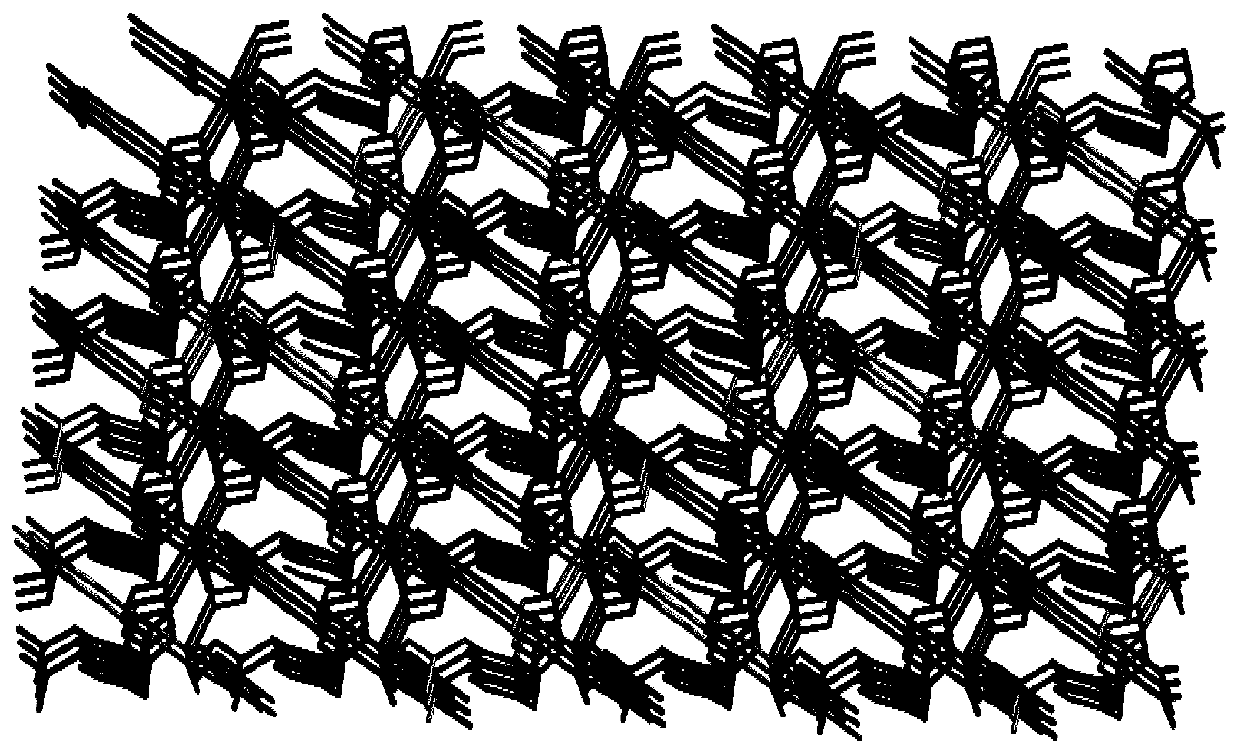
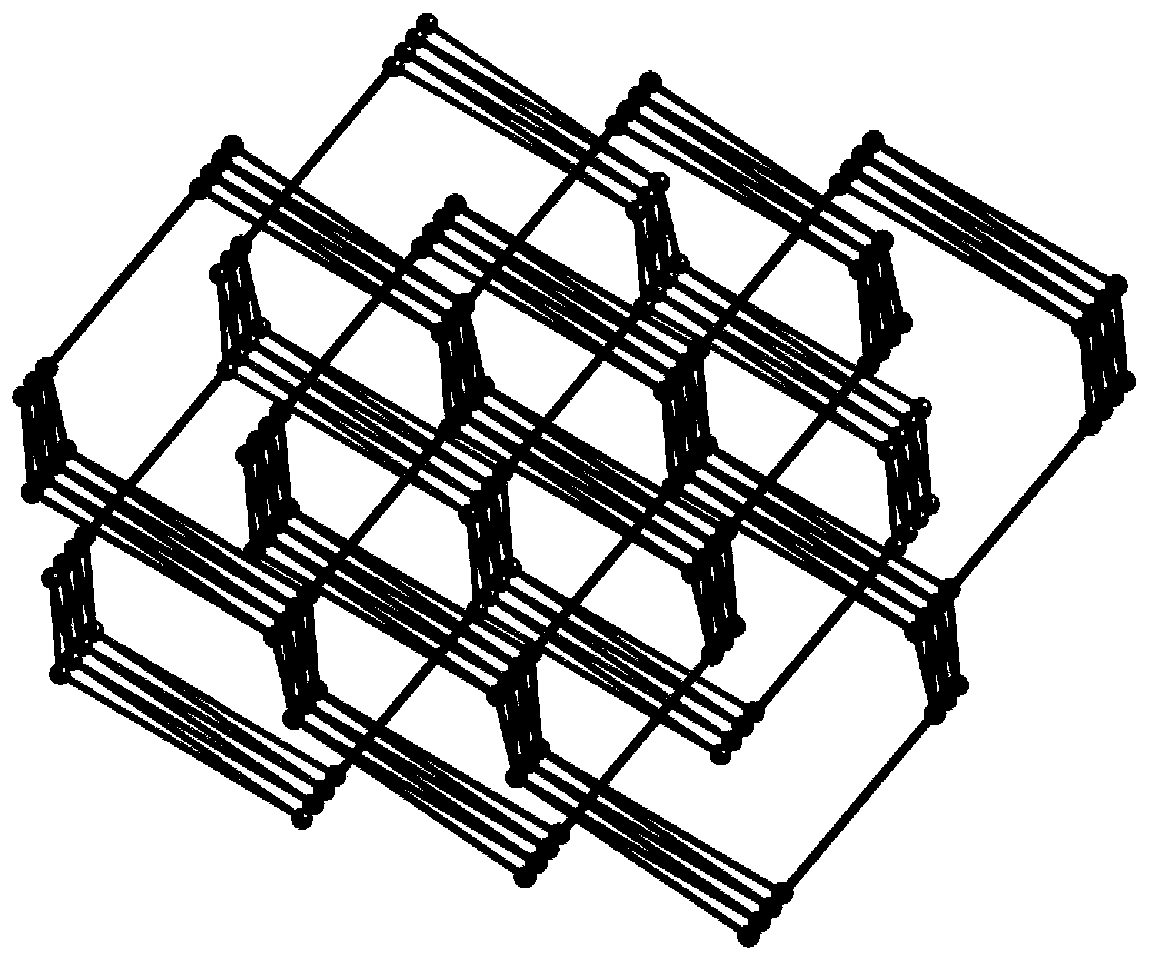
[0043] (1) Determination of the crystal structure of the complex
[0044] A single crystal with...
Embodiment 2
[0058] Dissolve 5-nitroisophthalic acid (21.1mg, 0.1mmol), potassium hydroxide (11.2mg, 0.2mmol), BTB (24.1mg, 0.1mmol) and cadmium perchlorate (41.9mg, 0.1mmol) in Water (10mL), sealed in a 16mL reaction kettle, heated to 170°C at a rate of 10°C per hour, maintained at this temperature for 3 days, and then cooled to room temperature naturally, to obtain colorless blocky crystals, which were separated After washing and drying in sequence, the target product is obtained with a yield of about 55%.
Embodiment 3
[0060] Dissolve 5-nitroisophthalic acid (21.1mg, 0.1mmol), potassium hydroxide (11.2mg, 0.2mmol), BTB (24.1mg, 0.1mmol) and cadmium perchlorate (25.4mg, 0.06mmol) in Water (10mL), sealed in a 16mL reaction kettle, heated to 180°C at a rate of 10°C per hour, maintained at this temperature for 3 days, and then cooled to room temperature naturally, to obtain colorless blocky crystals, which were separated After washing and drying in sequence, the target product is obtained with a yield of about 45%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com