Single-domain antibody specifically for MMP9 protein ZnMc structural domain as well as product and application thereof
A single-domain antibody, MMP-9 technology, applied in the field of biomedicine, can solve the problems of incomplete specificity and efficacy, small modification space, and high immune heterogeneity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
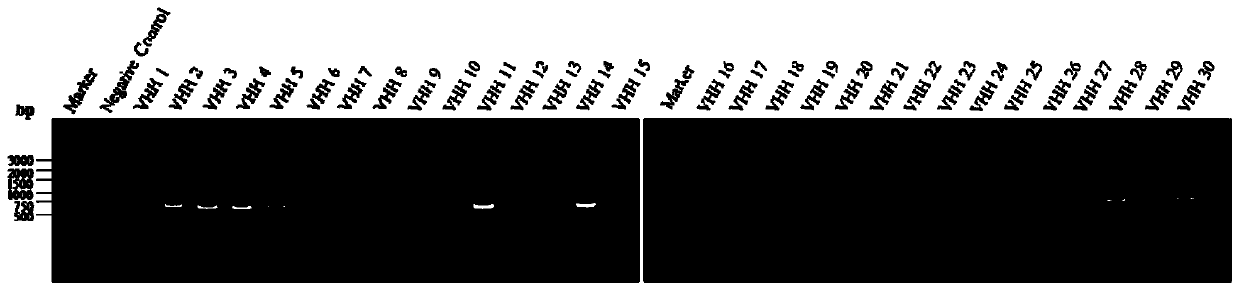
[0090] Example 1: Construction of a single domain antibody library against MMP-9 protein:
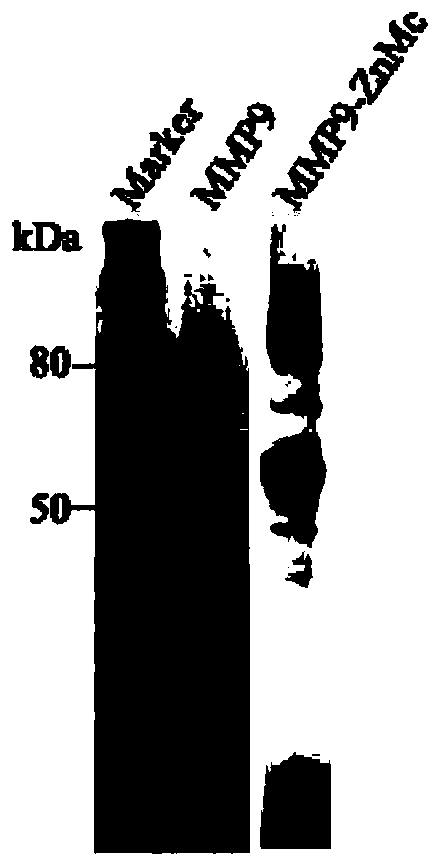
[0091] (1) The protein ZnMc of 1mg MMP-9 zinc ion binding domain is mixed with Freund's complete adjuvant of equal volume (the purity detection result of the protein for immunization is as follows figure 1 shown), immunize a Bactrian camel in Alxa, Inner Mongolia, after the first immunization, use protein ZnMc mixed with an equal volume of Freund's incomplete adjuvant for immunization, immunize once a week, and then immunize 4 times consecutively, a total of 5 consecutive immunizations ; Then in the 6th and 7th times, animals were immunized with 1 mg MMP-9 full-length protein mixed with Freund's incomplete adjuvant in equal volumes. This immunization process was to stimulate the camels to produce antibodies against the ZnMc domain. This domain can activate the metalloprotease activity of MMP-9 after binding with Zn, and the protease activity can be blocked by obtaining an antibody that ...
Embodiment 2
[0094] Example 2: Screening against MMP-9 protein single domain antibody:
[0095] (1) Take 200 μL of recombinant TG1 cells to culture in 2×TY medium, add 40 μL of helper phage VCSM13 to infect TG1 cells during the period, and culture overnight to amplify the phages, use PEG / NaCl to precipitate the phages the next day, and centrifuge to collect the amplified phages ;
[0096] (2) NaHCO diluted in 100mM pH 8.3 3 500 μg of the ZnMc protein in the medium was coupled to the microtiter plate, placed overnight at 4°C, and a negative control well was set up at the same time;
[0097] (3) Add 200 μL of 3% skim milk the next day, and block at room temperature for 2 hours;
[0098] (4) After blocking, add 100 μl of amplified phage library (approximately 2×10 11 phage particles), at room temperature for 1 h;
[0099] (5) After acting for 1 hour, wash 5 times with PBS+0.05% Tween-20 to wash away unbound phage;
[0100] (6) Use trypsin at a final concentration of 25 mg / mL to dissociat...
Embodiment 3
[0101] Embodiment 3: use the enzyme-linked immunosorbent method (ELISA) of phage to screen the specific positive clone against MMP-9:
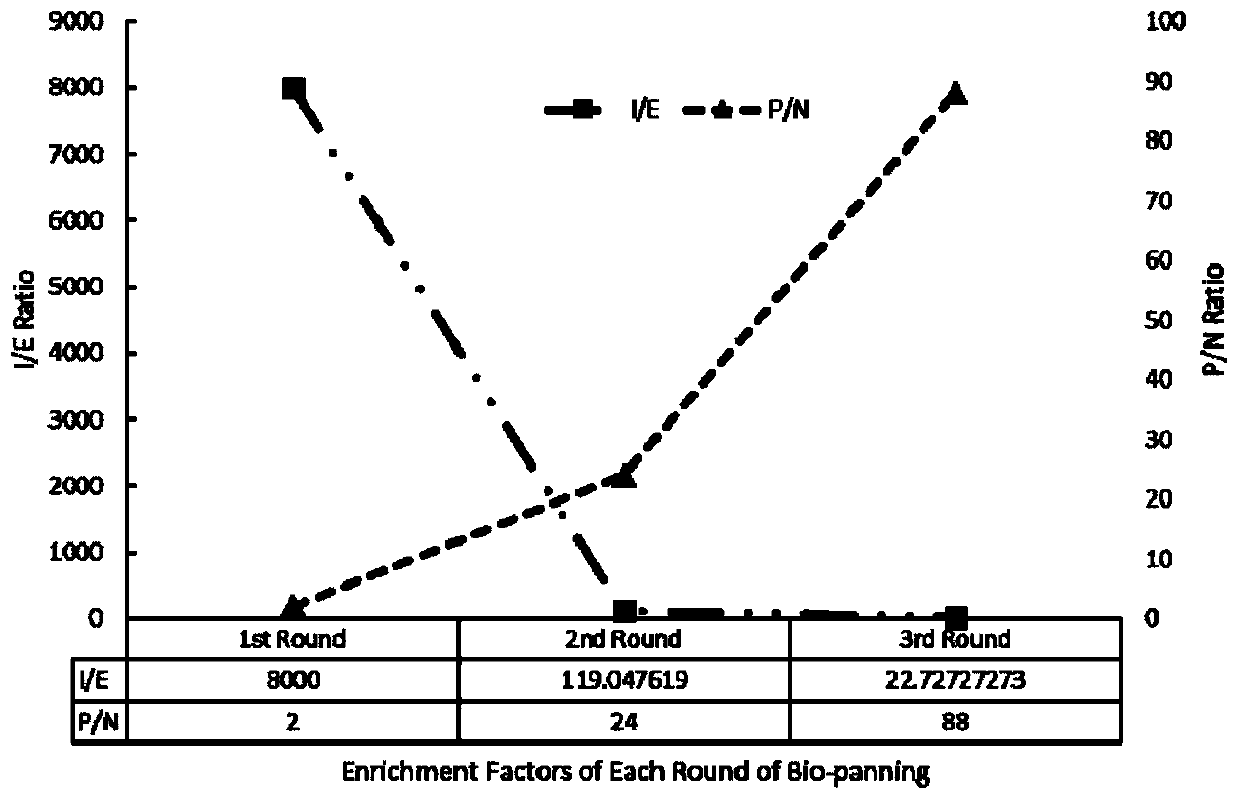
[0102] (1) According to the above-mentioned single-domain antibody screening method, three rounds of screening were performed on the MMP-9 protein. After the screening, the phage enrichment factor for the MMP-9 protein reached more than 10 (about 10,000), and the positive clones obtained from the screening Select 400 single colonies and inoculate them in 96-deep-well plates of TB medium containing 100 μg / mL ampicillin, and set up a blank control. After culturing to the logarithmic phase at 37°C, add IPTG with a final concentration of 1mM and culture at 28°C overnight;
[0103] (2) Use the osmotic swelling method to obtain the crude antibody; dilute the full-length MMP-9 protein and ZnMc protein to 100mM NaHCO at pH 8.3 3 Medium and coat 100 μg of protein in a microtiter plate overnight at 4°C;
[0104] (3) Transfer 100 μL of the antibody cru...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap