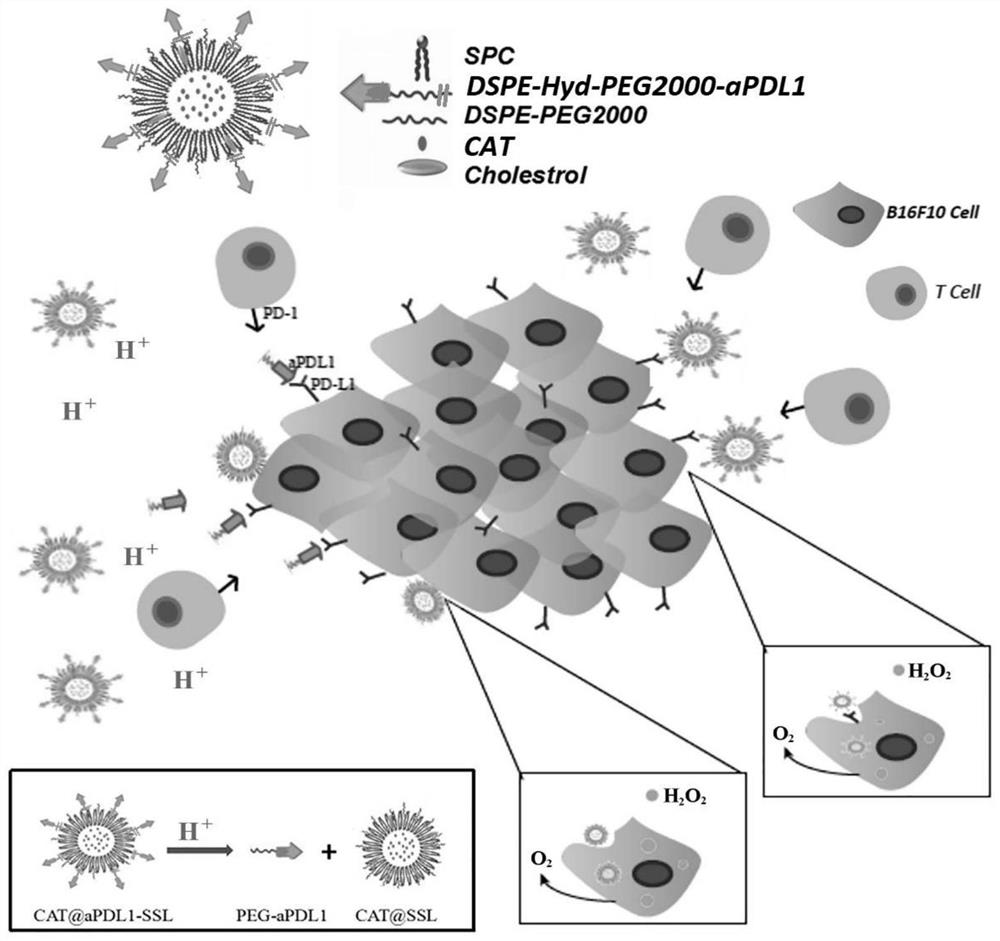
A liposome carrying catalase and linked to PD-L1 antibody and its preparation method
A catalase and liposome technology, applied in biochemical equipment and methods, oxidoreductase, liposome delivery and other directions, can solve the problems of increasing difficulties in clinical application, reducing toxic and side effects, and increasing the number of administrations, etc. To achieve the effect of promoting the killing of tumor cells, enhancing the effect of immunotherapy, and relieving tumor hypoxia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1 liposome preparation
[0056] Ordinary long-circulating liposomes (SSL) are prepared by thin-film dispersion method.
[0057] Raw material SPC (soybean lecithin), cholesterol, DSPE-PEG2000, dissolved in 1ml chloroform, the molar ratio is 100:50:8. Afterwards, the chloroform solution was suspended to dryness using a rotary evaporator, and the chloroform solution was removed to obtain a lipid film. The lipid film was hydrated with 2 ml of phosphate buffered saline (PBS). The final SSL was obtained after filtration through a 200nm polycarbonate membrane.
[0058] Multifunctional immunoliposomes (CAT@aPDL1-SSL) were prepared by thin-film dispersion / post-insertion method.
[0059] First, the raw material-directed compound DSPE-Hyd-PEG2000-NHS is mixed with aPDL1 at a molar ratio of 10:1, and the aPDL1-directed compound DSPE-Hyd-PEG2000 is synthesized by reacting the amino group of the antibody aPDL1 with the succinimide group of the raw material-directed co...
Embodiment 2
[0061] The characterization of embodiment 2 liposome
[0062] 1. Experimental process
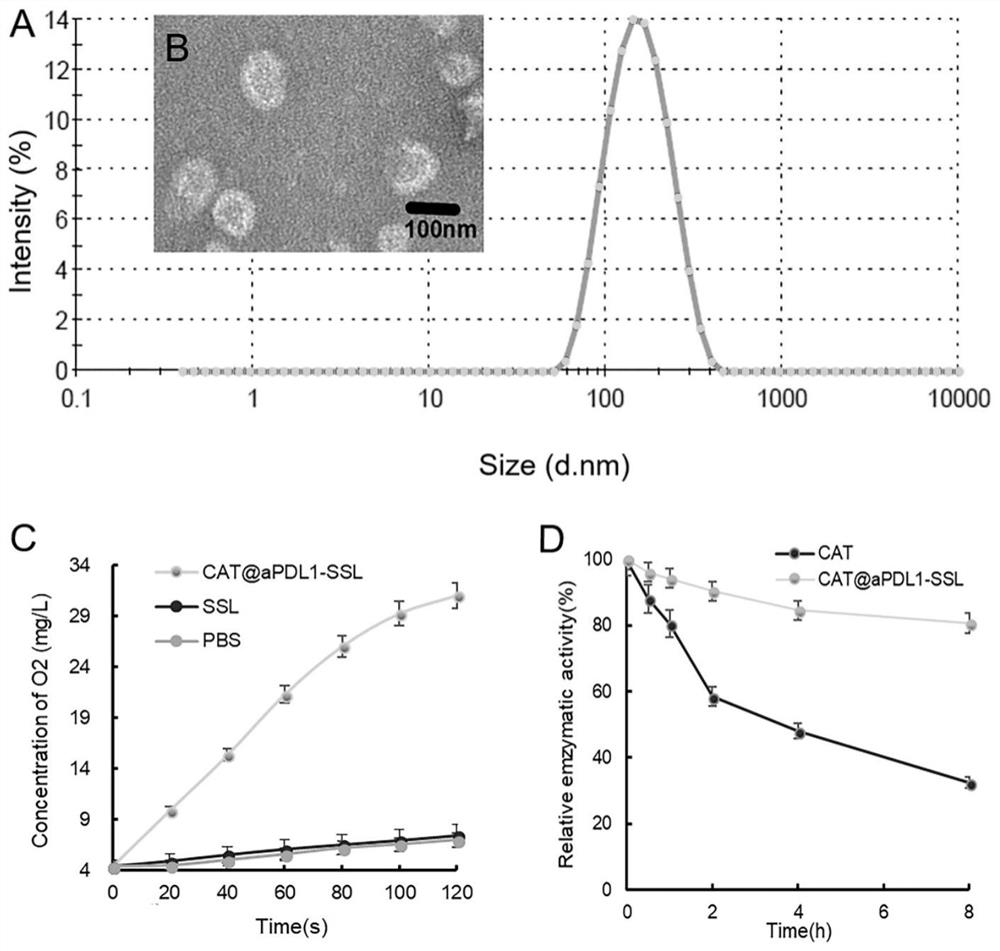
[0063] The particle size and dispersion of CAT@aPDL1-SSL were measured by a dynamic light scattering particle sizer (DLS) (Zetasizer Nano ZS90; Malvern; UK).
[0064] After the liposome solution in Example 1 was stained with 2% phosphotungstic acid, the morphology of the particles was observed with a transmission electron microscope (TEM) (JEM-1400Plus, JEOL, Japan).
[0065] The entrapment efficiency of CAT was measured by BCA protein quantification.
[0066] Enzyme activity of free CAT and CAT entrapped in liposomes was determined by standard Goth's method.
[0067] First, 0.5 mL of H 2 O 2 The solution (30% aqueous solution) was added to 1.5 mL of Eppendorf (EP) tubes, then 1 mL of free CAT and 1 mL of CAT@aPDL1-SSLs were added to each EP tube and incubated at 37 °C with H 2 O 2 React for 1 minute.
[0068] Subsequently, 0.5 mL of ammonium molybdate (32.4 mM) was added to the re...
Embodiment 3
[0083] Example 3 In vitro cell uptake experiment
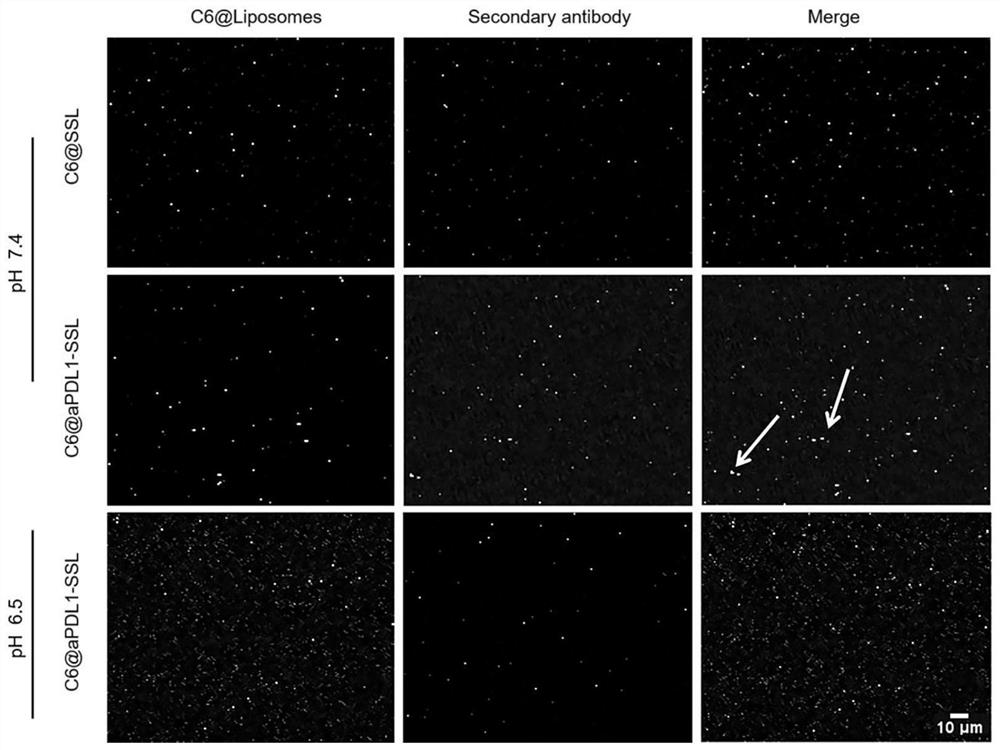
[0084] 1. Prepare C6-loaded liposome C6@aPDL1-SSL using the above-mentioned film dispersion method.
[0085] B16-F10 cells in 1 × 10 6 Cells / well density were seeded into six-well plates and incubated overnight. The medium was then removed, and the cells were washed 3 times with PBS. Add PBS, free C6, C6@aPDL1-SSL, C6@aPDL1-SSL and free aPDL1 (pH 7.4) to each well of the plate (C6, 150ng / mL) and mix with B16-F10 cells at 37°C and 5% CO 2 Incubate for 2 hours under conditions. After 2 hours, the sample solution was removed, and 200 μL of trypsin and ethylenediaminetetraacetic acid (EDTA) were added to each well to digest the cells. The digested cell suspension was centrifuged at 1,000rpm for 5 minutes, and then the pellet was washed at 1×10 6 Resuspend in PBS at a density of cells / mL.
[0086] To study the cellular uptake of liposomes under low pH conditions, the medium was adjusted to pH 6.5 using citrate buffer (pH 6...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com