Green preparation process of m-tert-butylphenol
A technology for m-tert-butylphenol and tert-butylphenol, which is applied in the field of green preparation technology of m-tert-butylphenol, can solve the problems of serious environmental pollution, many by-products and high yield, and achieves simple process, economic benefits and high yield. Improved social benefits and good atomic economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
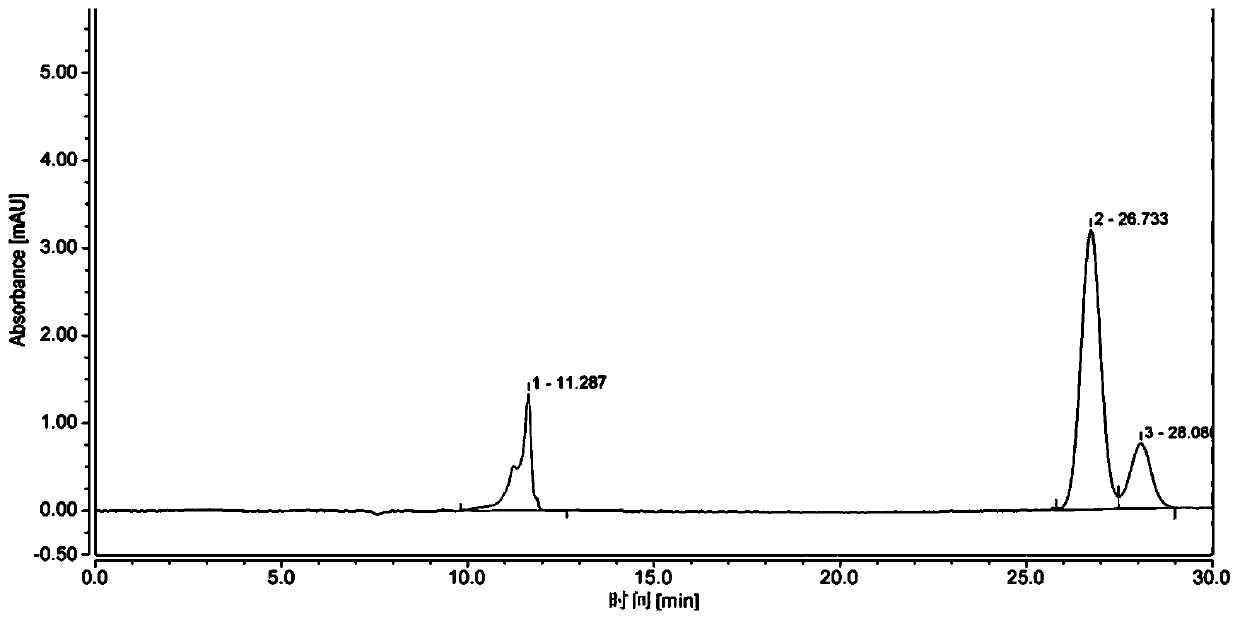
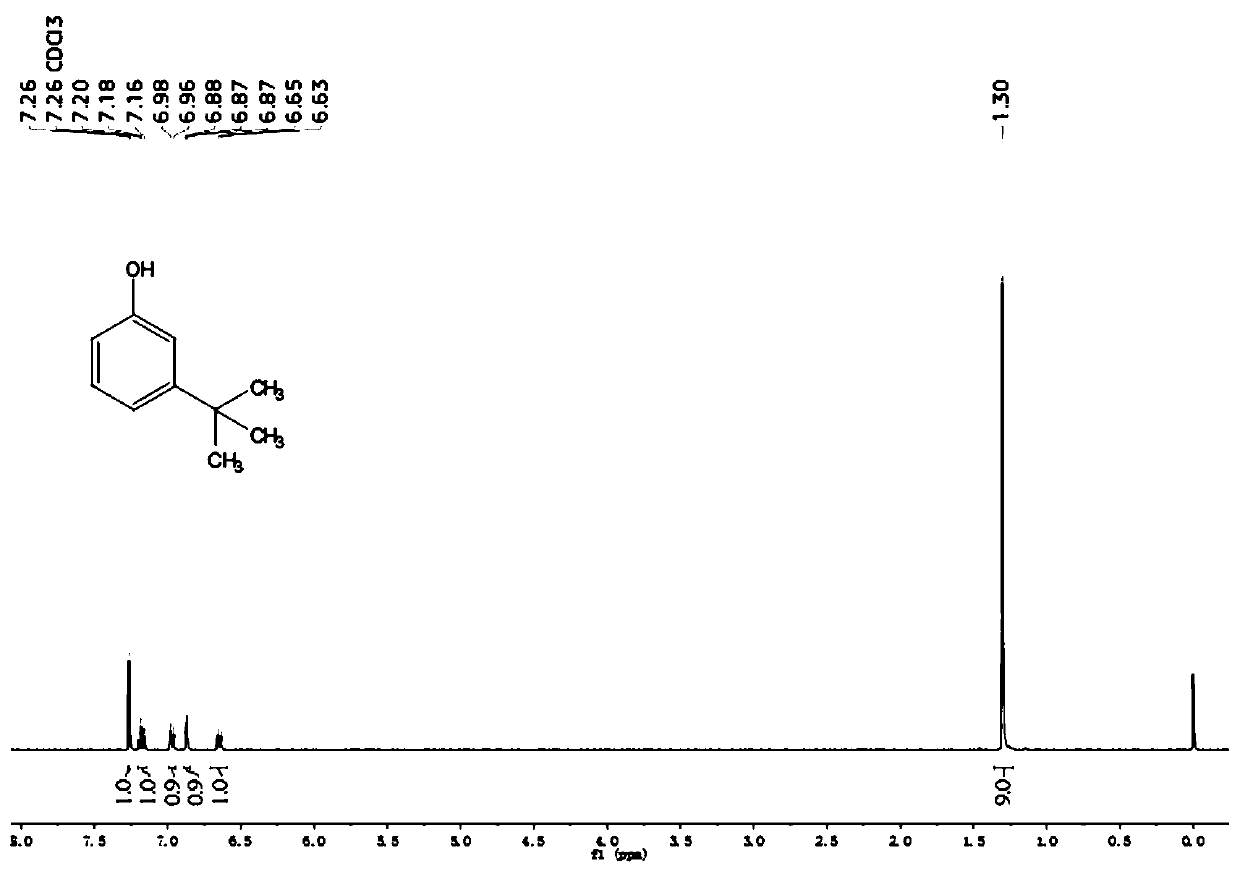
[0024] (1) 10g of phenol, 0.1g of clay and 50mg of sulfuric acid are added to the round-bottomed reaction flask, the reaction temperature is controlled at 150°C, and 15ml of tert-butyl chloride is added dropwise, the dropwise is completed, and stirred for 3 hours. After the reaction is completed, Filter out the catalyst, pour the reaction solution into 100ml of water to obtain an organic phase layer and an inorganic phase layer, add 300ml ethyl acetate to extract three times, combine to obtain an organic phase layer solution, and the organic phase layer solution is washed with anhydrous NaSO 4 Dry, recover ethyl acetate, distill under reduced pressure, collect 125~130°C / 20mmHg fractions to obtain m-tert-butylphenol with a purity of 98.0%, such as figure 2 as shown, 1 H NMR (400MHz, CDCl 3 )δ7.26(s,1H),7.18(t,J=8.0Hz,1H),6.97(d,J=7.8Hz,1H),6.89–6.85(m,1H),6.64(d,J=8.4 Hz, 1H), 1.30 (s, 9H), liquid chromatography retention time t = 26.733min (methanol: water = 1:1), collect 1...
Embodiment 2
[0028] (1) 20g phenol, 0.17g clay and 75mg sulfuric acid are added in the round-bottomed reaction flask, and the reaction temperature is controlled at 150°C, and 19ml tert-butyl chloride is added dropwise, and the dropwise is completed and stirred for 3 hours. After the reaction is completed, Filter out the catalyst, pour the reaction solution into 200ml of water to obtain an organic phase layer and an inorganic phase layer, add 400ml ethyl acetate to extract three times, combine to obtain an organic phase layer solution, and the organic phase layer solution is washed with anhydrous NaSO 4 Dry, recover ethyl acetate, distill under reduced pressure, collect fractions at 125-130°C / 20mmHg to obtain m-tert-butylphenol with a purity of 97.1%, collect fractions at 110-115°C / 10mmHg to obtain p-tert-butylphenol, collect 82- 87 ° C / 20mmHg fraction, phenol.
[0029] (2) 1.9g aluminum chloride and 0.14g white clay are added in the round-bottomed reaction flask, add the 10g p-tert-butyl...
Embodiment 3
[0031] (1) 35g phenol, 0.28g clay and 115mg sulfuric acid are added in the round-bottomed reaction flask, and the reaction temperature is controlled at 150°C, and 29ml of tert-butyl chloride is added dropwise, and the dropwise is completed and stirred for 3 hours. After the reaction is completed, Filter out the catalyst, pour the reaction solution into 400ml of water to obtain an organic phase layer and an inorganic phase layer, add 600ml ethyl acetate to extract three times, combine to obtain an organic phase layer solution, and the organic phase layer solution is washed with anhydrous NaSO 4 Dry, recover ethyl acetate, distill under reduced pressure, collect fractions at 125-130°C / 20mmHg to obtain m-tert-butylphenol with a purity of 98.3%, collect fractions at 110-115°C / 10mmHg to obtain p-tert-butylphenol, collect 82- 87 ° C / 20mmHg fraction, phenol.
[0032] (2) 2.6g aluminum chloride and 0.19g white clay are added in the round-bottomed reaction flask, add the 15g p-tert-b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com