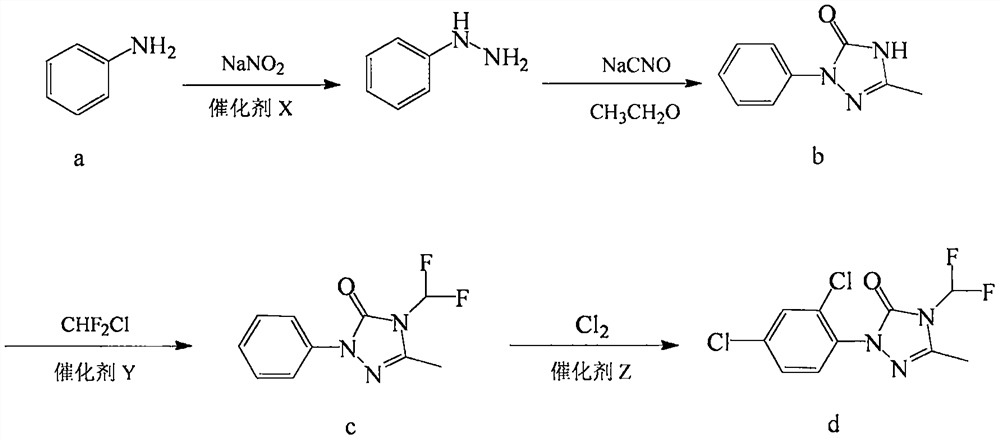
Preparation method of 1-(2,4-dichlorophenyl)-4-difluoromethyl-3-methyl-1H-1,2,4-triazole-5-one
A technology of difluoromethyl and dichlorophenyl, applied in the field of chemical preparation, can solve the problems of complex treatment process, unfavorable environmental protection, excessive waste water, etc., achieves cheap and easy-to-obtain process raw materials, is conducive to environmental protection treatment, and simplifies the process effect of operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Preparation of Catalyst X: Dissolve 3.72g (0.0196mol) of tin dichloride and 0.057g (0.0004mol) of germanium dichloride in 20g of water with a pH value of 3 at room temperature, and use as needed.
[0038] Step 1, mix 93.1g (1mol) aniline a with 148g (1.5mol) 37% concentrated hydrochloric acid to generate aniline hydrochloride solution, cool to 0°C and add dropwise 207g (1.2mol) 40% sodium nitrite aqueous solution, Add all the catalyst X solution prepared dropwise at 0-5°C, react at room temperature for 6 hours, and adjust the pH value of the reaction solution to 11-12 with 30% sodium hydroxide solution. Add 186.2g tert-butanol, add 104.7g (0.95mol) 40% acetaldehyde aqueous solution dropwise at 0-5°C, add 65g (1mol) sodium cyanate, and add 60.1g (1mol) acetic acid dropwise after the reaction solution is clear, below room temperature Stir for 3h, drop the temperature to 0-5°C and add 706.8g (0.95mol) of 10% sodium hypochlorite solution dropwise, stir for 2h, filter, wash ...
Embodiment 2
[0044] The preparation of catalyst X, step 1, the preparation of catalyst Y, the preparation of catalyst Z and step 3 are the same as in Example 1.
[0045] Wherein step 2 is: put 151.8g (0.85mol) 1-phenyl-3-methyl-1H-1,2,4-triazol-5-one b, 2.5g of the catalyst Y into 203.6g of acetonitrile, Introduce nitrogen to replace the air in the reaction system, stir and heat up to 60°C, pass in 110.7g (1.28mol) of chlorodifluoromethane within 5 hours, filter after cooling down to room temperature, wash and filter with 151.8g ethylene glycol monomethyl ether Cake, combined filtrates, precipitation to obtain 187g of reddish-brown solid, 1-phenyl-3-methyl-4-difluoromethyl-1H-1,2,4-triazol-5-one c content 95.2% , The yield was 93.1% based on 1-phenyl-3-methyl-1H-1,2,4-triazol-5-one b.
Embodiment 3
[0047] The preparation of catalyst X, step 1, the preparation of catalyst Z and step 3 are the same as in Example 1.
[0048] The preparation of catalyst Y is as follows: 10.7g (0.255mol) sodium fluoride and 10.7g diatomite (325 mesh, specific surface area 600m 2 / g) into 21.4g of water, stir evenly, remove water by rotary evaporation, vacuum dry, and set aside.
[0049] Step 2 is to put 151.8g (0.85mol) of 1-phenyl-3-methyl-1H-1,2,4-triazol-5-one b and 2g of the catalyst Y into 203.6g of acetonitrile, and replace with nitrogen The air in the reaction system was stirred and the temperature was raised to 60°C. 88.2g (1.02mol) of chlorodifluoromethane was introduced within 5h, and filtered after cooling down to room temperature. The filter cake was washed with 152g of ethylene glycol monomethyl ether, and the filtrates were combined. Precipitate to obtain 187g of reddish-brown solid, 1-phenyl-3-methyl-4-difluoromethyl-1H-1,2,4-triazol-5-one c content of 95.1%, based on 1-benzen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com