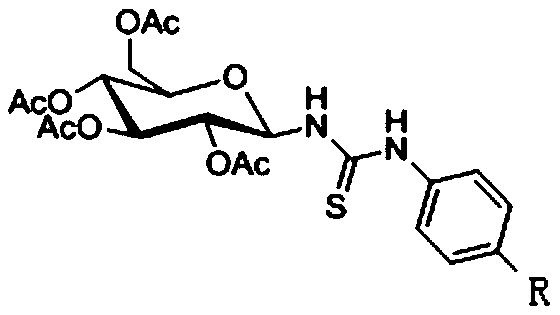
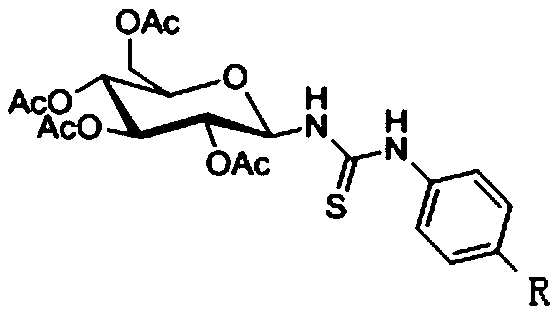
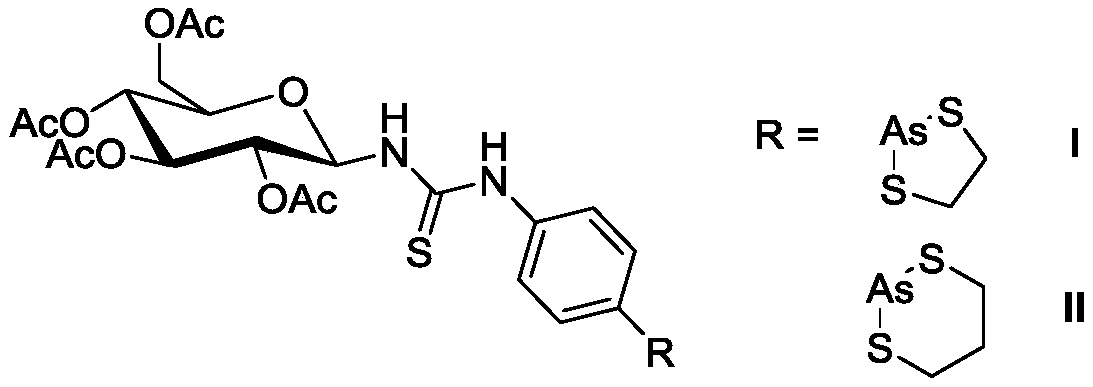
Thiourea-containing arsenic sugar with anti-tumor activity and preparation method and application thereof
An anti-tumor activity, thiourea arsenic sugar technology, applied in the field of arsenic sugar, can solve the problems of reduced yield, easy oxidation of intermediates, etc., and achieves the effects of simple reaction operation, high yield and good anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1-(N-(4'-(1",3",2"-dithiarsen-2-yl)phenyl)-thiourea)-2,3,4,6-O-acetyl - Preparation of β-D-glucose (I):
[0032] 1) Preparation of 4-aminophenyl arsenous acid
[0033] a. Weigh 4-aminophenylarsenic acid (5g, 23mmol), potassium iodide (25mg, 0.15mmol) and dissolve it in a mixed solution of methanol / concentrated hydrochloric acid (30mL / 24mL), cool down to 0°C, and add SO 2 Gas for 30min until the solution turns from orange red to light yellow. The solution was kept in the refrigerator at 0°C overnight, filtered with suction, and the filter cake was washed twice with 25 mL of cold diethyl ether to obtain 4-dichloroarsenoaniline hydrochloride (6.2 g, 93.7%);
[0034] b. Dissolve 4-dichloroarsenoaniline hydrochloride (6.2g, 93.7%) in 150mL aqueous solution, cool down to 0-5°C, add 10 mol / L concentrated ammonia water in batches, to a total of 26mL, stir for 30 minutes, A white solid was precipitated, the solution was kept at 0-5°C overnight, filtered, the filter cake was w...
Embodiment 2
[0046] 1-(N-(4'-(1",3",2"-dithiarsen-2-yl)phenyl)-thiourea)-2,3,4,6-O-acetyl - Preparation of β-D-glucose (II):
[0047] 1) Preparation of 4-aminophenyl arsenous acid
[0048] a. Weigh 4-aminophenylarsenic acid (5g, 23mmol), potassium iodide (25mg, 0.15mmol) and dissolve it in a mixed solution of methanol / concentrated hydrochloric acid (30mL / 24mL), cool down to 0°C, and add SO 2 Gas for 30min until the solution turns from orange red to light yellow. The solution was kept in the refrigerator at 0°C overnight, filtered with suction, and the filter cake was washed twice with 25 mL of cold diethyl ether to obtain 4-dichloroarsenoaniline hydrochloride (6.2 g, 93.7%);
[0049] b. Dissolve 4-dichloroarsenoaniline hydrochloride (6.2g, 93.7%) in 150mL aqueous solution, cool down to 0-5°C, add 10 mol / L concentrated ammonia water in batches, to a total of 26mL, stir for 30 minutes, A white solid was precipitated, the solution was kept at 0-5°C overnight, filtered, the filter cake was ...
Embodiment 3
[0061] Example 3 Influence of the above-mentioned compounds I and II on the inhibitory effect of liver cancer cells HepG2
[0062] experimental method:
[0063] 1. Dilute hepatoma cell HepG2 to 1×10 6 / mL of cell suspension, take 100uL and inoculate in a 96-well plate
[0064] 2. After the cells adhered to the wall, the medium (DMEM, 10% FBS) was aspirated, and different concentrations of compound I or II were added to the 96-well plate (medium dilution), and the culture was continued for 48 hours.
[0065] 3. Add 10uL (5mg / mL) MTT solution (diluted in PBS) to each well and continue to incubate for 4h
[0066] 4. Aspirate the medium and add 150uL DMSO to each well
[0067] 5. Shake on a shaker for 10 minutes, and detect the absorbance at 490nm with a microplate reader
[0068] 6. Cell survival rate=experimental group) / blank group (blank group: no drug added), each group was parallelized three times, and the IC was calculated 50 .
[0069] Compound I:IC 50 = 7.62 ± 0.10 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com