Silane modified polylactic acid as well as preparation method and application thereof
A technology of silane-modified polylactic acid, which is applied in the direction of adhesives, etc., can solve problems such as the application of silane-modified polylactic acid that have not yet appeared, and achieve the effects of avoiding adverse effects, improving flexibility and hydrophilicity, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
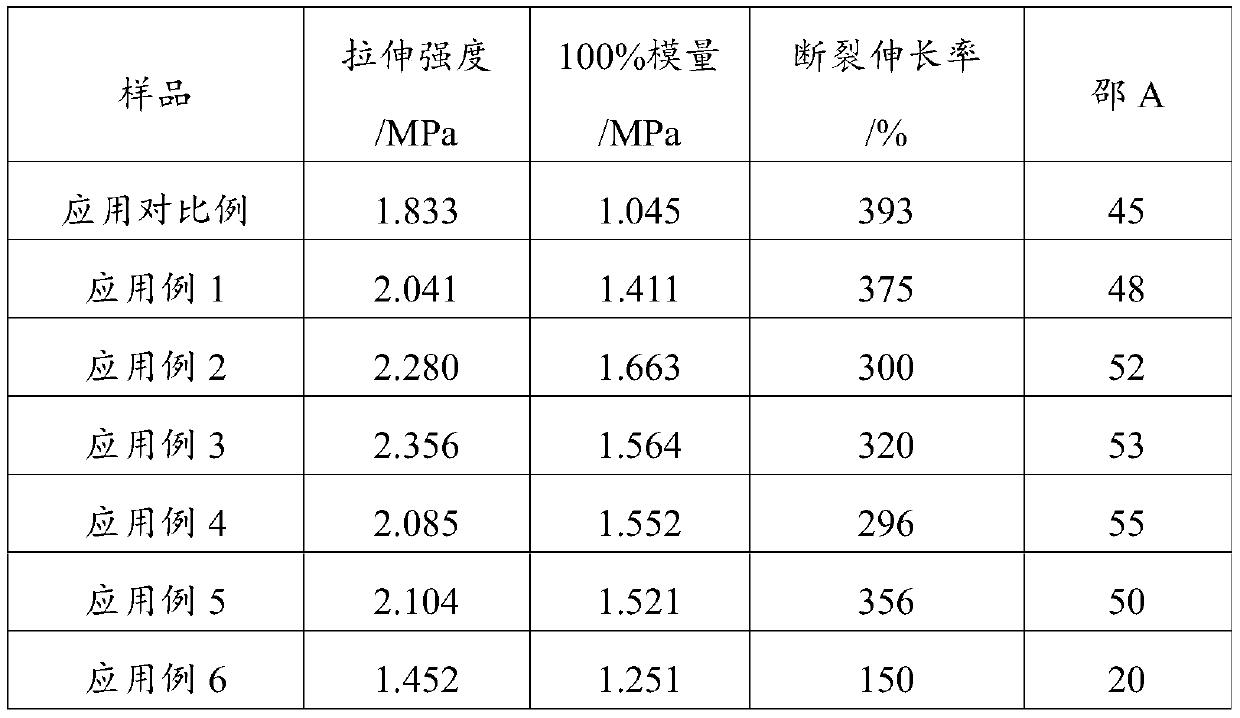
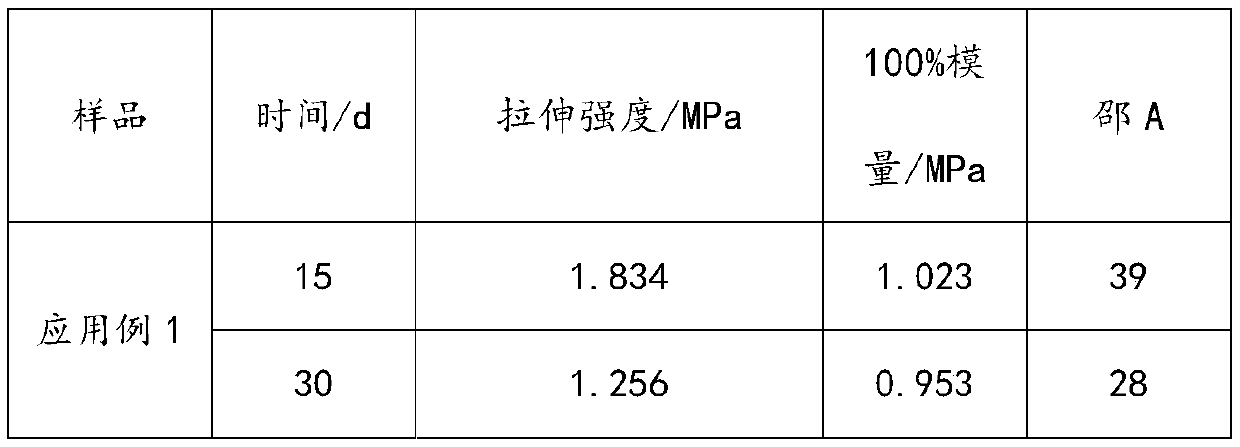
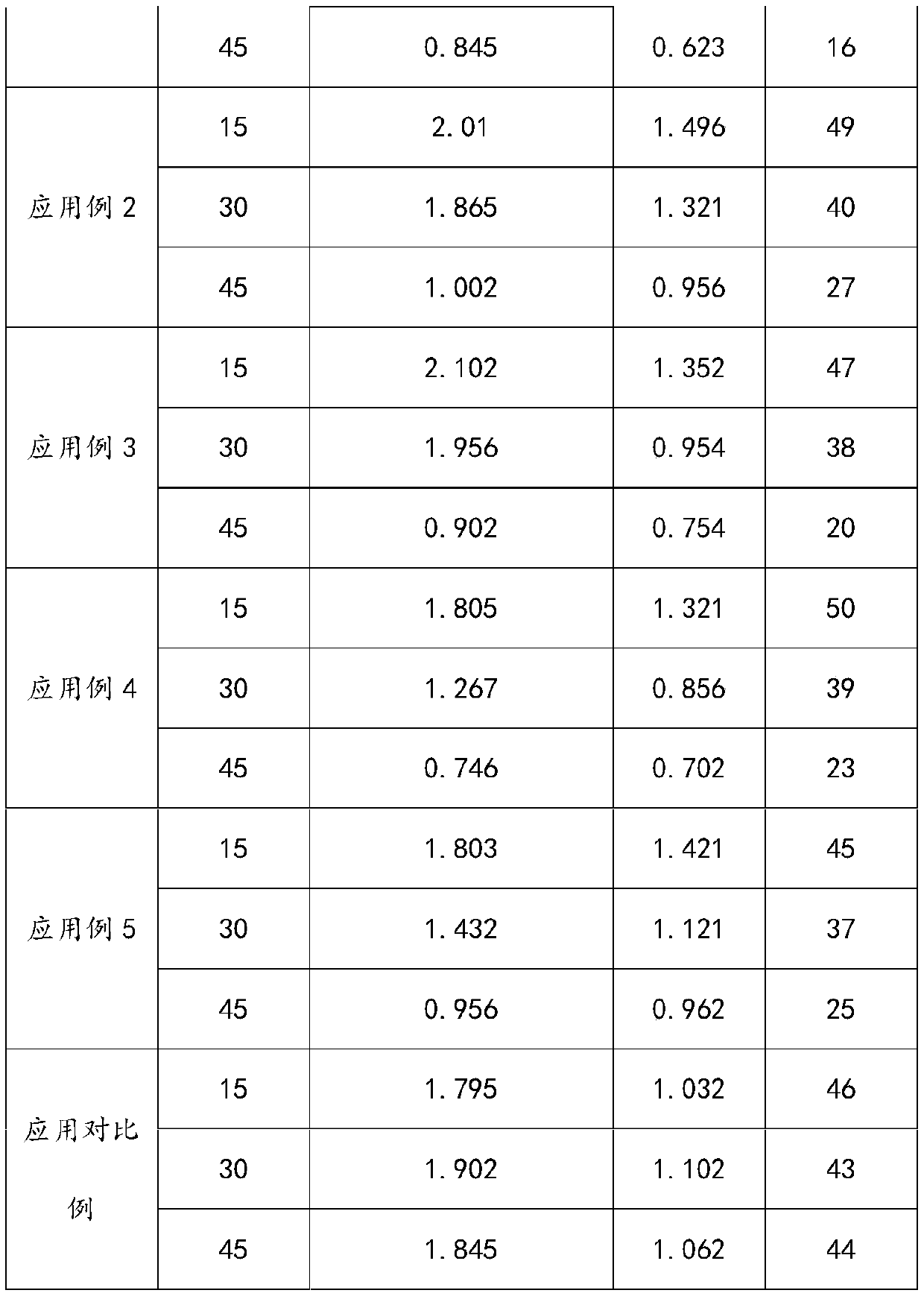
Embodiment 1
[0035]1) Add 80.4g of polyether polyol with a number average molecular weight of 300 (1,2-propylene glycol, PO homopolymerization), 325.5g of lactide, and 2.0g of stannous octoate into a three-necked flask, start stirring and vacuumize 1. Nitrogen replacement was alternated 5 times, and the temperature was raised to 140° C. under the protection of nitrogen, and kept at this temperature for 12 hours to obtain 403.8 g of polylactic acid polyols with an acid value of 4.2 mgKOH / g.
[0036] 2) Take 300.3g of the above-mentioned polylactic acid polyol and add 0.3g of ferric chloride into a pressure-resistant reaction kettle, start stirring, vacuumize, alternate nitrogen replacement for 5 times, then heat up to 120°C, add 5.6g of propylene oxide (for poly 4.3 times the number of moles of carboxyl groups of lactic acid polyols), pressurize to 0.5MPa with nitrogen, keep warm at 120°C for 3.5h, then vacuumize to remove unreacted propylene oxide, cool down to below 40°C to obtain polylact...
Embodiment 2
[0039] 1) Add 200.3 g of polyether polyols with a number-average molecular weight of 1000 (glycerin starts, PO, EO random copolymerization, EO mass content in polyether segments is 15%), 400.7 g of lactide, in a three-necked flask, 3.0g of stannous octoate, start stirring, vacuumize and replace with nitrogen 5 times alternately, raise the temperature to 135°C under the protection of nitrogen, and keep the temperature for 15h to obtain 596.7g of polylactic acid polyol with an acid value of 3.7mgKOH / g.
[0040] 2) Take 450.7g of the above-mentioned polylactic acid polyol, add 3.2g of chromium acetate into a pressure-resistant reactor, start stirring, vacuumize, alternate nitrogen replacement for 5 times, then heat up to 85°C, add 4.8g of propylene oxide (polylactic acid polyol 2.8 times the number of moles of carboxyl groups of alcohol), pressurize with nitrogen to 0.27MPa, keep warm at 85°C for 3.5h, then vacuumize to remove unreacted propylene oxide, cool down to below 40°C a...
Embodiment 3
[0043] 1) Add 125.6g of polyether polyol (starting from ethylene glycol, EO homopolymerization) with a number average molecular weight of 800, 500.9g of lactide, and 3.2g of stannous octoate into a three-necked flask, start stirring, vacuumize, and nitrogen The replacement was alternated 5 times, and the temperature was raised to 140° C. under the protection of nitrogen, and kept at this temperature for 14 hours to obtain 623.2 g of polylactic acid polyol with an acid value of 3.5 mgKOH / g.
[0044] 2) Take 500g of the above-mentioned polylactic acid polyol, add 7.5g of ferric chloride into a pressure-resistant reactor, start stirring, then vacuumize, alternate nitrogen replacement for 5 times, then raise the temperature to 50°C, add 1.4g of ethylene oxide (for poly The carboxyl molar number of lactic acid polyol is 1 times), nitrogen pressurization to 0.5MPa, heat preservation at 50°C for 8 hours, vacuumize to remove unreacted ethylene oxide, cool down to below 40°C and take sa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| acid value | aaaaa | aaaaa |
| acid value | aaaaa | aaaaa |
| acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com