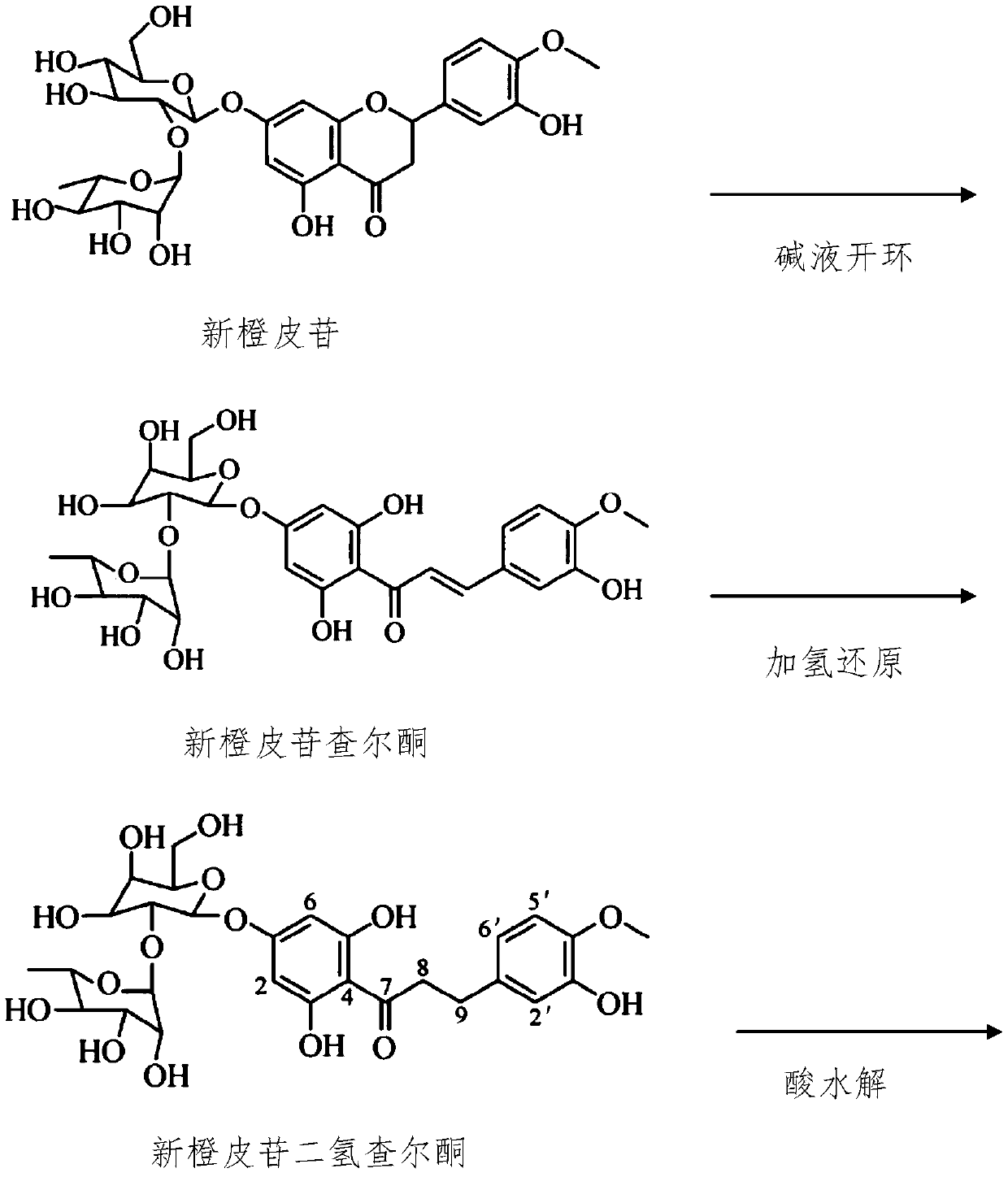
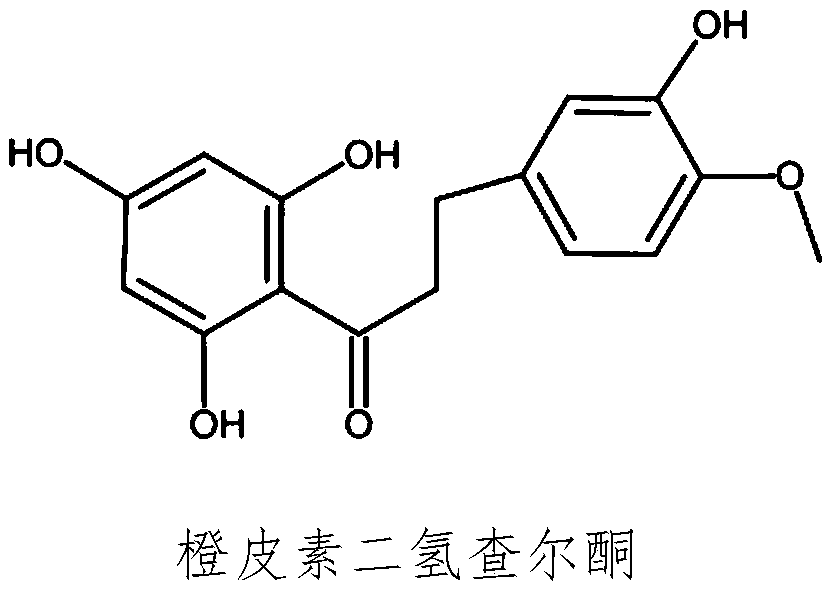
Synthesis method of hesperitin dihydrochalcone
A technology of neohesperidin dihydrochalcone and dihydrochalcone, which is applied in the field of synthesis of hesperetin dihydrochalcone, and can solve the problem of lack of resources and research on the synthesis process of hesperetin dihydrochalcone Few other problems, to achieve the effect of abundant sources, easy access, and pure taste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] A kind of synthetic method of hesperetin dihydrochalcone, comprises the following steps:
[0031] 1) Preparation of neohesperidin dihydrochalcone
[0032] Dissolve 100g of raw material neohesperidin in 1000g of sodium hydroxide solution with a mass concentration of 5%, then add 7g of ternary nickel catalyst, stir evenly, heat up to 40°C, and carry out hydrogenation reaction for 3h to obtain A solution of neohesperidin dihydrochalcone;
[0033] 2) Preparation of crude hesperetin dihydrochalcone
[0034] Use hydrochloric acid to adjust the pH of the solution containing neohesperidin dihydrochalcone to 2, heat up and reflux for 5 hours to complete hydrolysis, cool down to below 10°C, crystallize for 24 hours, filter and dry to obtain hesperetin dihydrochalcone crude ketone;
[0035] Step 3) prepare hesperetin dihydrochalcone boutique
[0036] The hesperetin dihydrochalcone crude product obtained in step 2) was recrystallized with 50% ethanol at 10 times its volume mass...
Embodiment 2
[0038] A kind of synthetic method of hesperetin dihydrochalcone, comprises the following steps:
[0039] 1) Preparation of neohesperidin dihydrochalcone
[0040] Dissolve 50g of raw material neohesperidin in 500g of sodium hydroxide solution with a mass concentration of 5%, then add 3.5g of ternary nickel catalyst, stir evenly, heat up to 40°C, and carry out hydrogenation reaction for 3h to obtain solutions containing neohesperidin dihydrochalcone;
[0041] 2) Preparation of crude hesperetin dihydrochalcone
[0042] Use hydrochloric acid to adjust the pH of the solution containing neohesperidin dihydrochalcone to 2, heat up and reflux for 5 hours to complete hydrolysis, cool down to below 10°C, crystallize for 24 hours, filter and dry to obtain hesperetin dihydrochalcone crude ketone;
[0043] Step 3) prepare hesperetin dihydrochalcone boutique
[0044] The hesperetin dihydrochalcone crude product obtained in step 2) was recrystallized with 50% ethanol at 10 times its volu...
Embodiment 3
[0046] A kind of synthetic method of hesperetin dihydrochalcone, comprises the following steps:
[0047] 1) Preparation of neohesperidin dihydrochalcone
[0048] Dissolve 150g of raw material neohesperidin in 1500g of sodium hydroxide solution with a mass concentration of 5%, then add 10.5g of ternary nickel catalyst, stir evenly, heat up to 40°C, and carry out hydrogenation reaction for 3h to obtain solutions containing neohesperidin dihydrochalcone;
[0049] 2) Preparation of crude hesperetin dihydrochalcone
[0050] Use hydrochloric acid to adjust the pH of the solution containing neohesperidin dihydrochalcone to 2, heat up and reflux for 5 hours to complete hydrolysis, cool down to below 10°C, crystallize for 24 hours, filter and dry to obtain hesperetin dihydrochalcone crude ketone;
[0051] Step 3) prepare hesperetin dihydrochalcone boutique
[0052] The hesperetin dihydrochalcone crude product obtained in step 2) was recrystallized with ethanol whose volume concentr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com