Method for preparing prostaglandin E1 by using gene engineering cyclooxygenase-1 and gene engineering prostaglandin E synthetase-1
A technology of prostaglandin and genetic engineering, applied in the field of preparing prostaglandin E1, which can solve the problems of reaction system pollution, large demand for fresh enzyme, excessive product impurities, etc., and achieve the effect that is beneficial to purification and utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
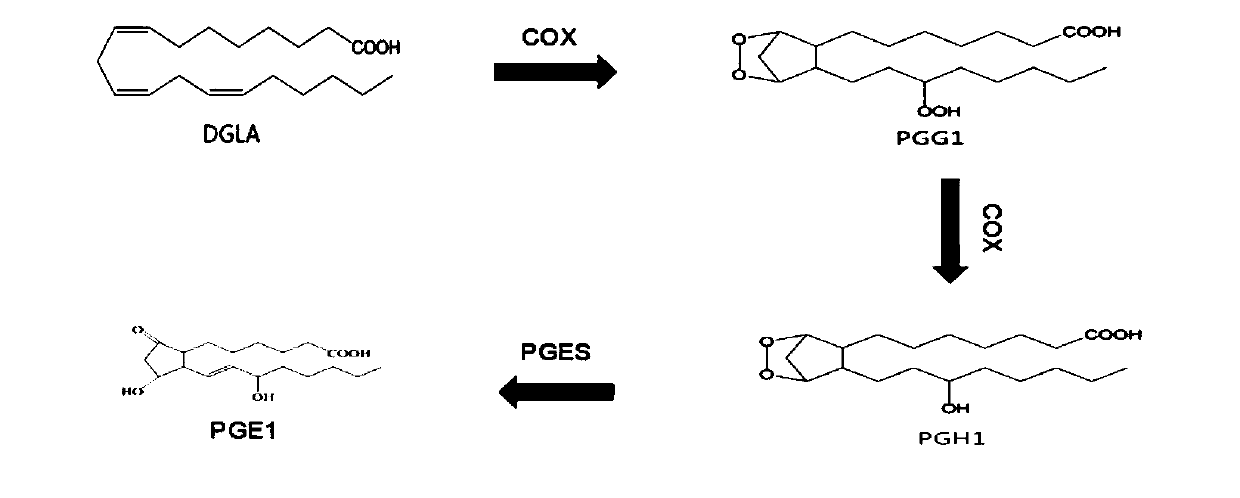
[0036] The synthetic mechanism of the preparation method of prostaglandin E1 provided by the invention is:
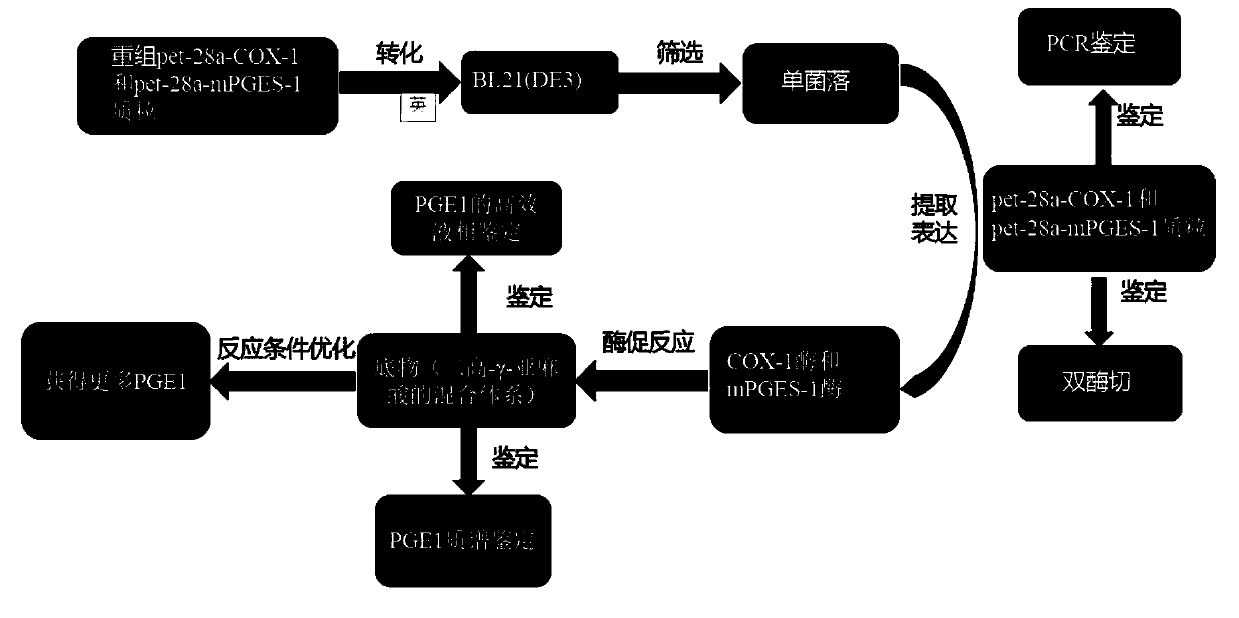
[0037] Dihomo-γ-linolenic acid (DGLA) is catalyzed by cyclooxygenase-1 (COX-1) to generate PGG1, hydrogen atoms are extracted from C-9 and C-11 of dihomo-γ-linolenic acid, and O 2 Form a C-9 / C11 endoperoxide with the generated carbon radical, form a carbon-carbon double bond through cyclization between C-8 and C-12, and then add an O to the C-15 position again 2 , and then form PGG1. PGG1 can then be reduced by the peroxidase activity of cyclooxygenase (thus in the presence of a reducing agent such as glutathione) to form PGH1 (PGH1 is an extremely unstable prostaglandin species). PGH1 is converted into PGE1 by prostaglandin E synthase-1 (mPGES-1). The theoretical synthesis pathway of prostaglandin E1 is as follows: figure 1 shown. The specific synthetic route of the present invention is as figure 2 shown.
Embodiment 1
[0039] Example 1 Recombinant plasmid construction and identification
[0040] Obtain the coding regions of the sheep cyclooxygenase-1 and prostaglandin E synthase-1 genes by searching in NCBI. The gene ID of cyclooxygenase-1 in NCBI is 443551, and the amino acid reference sequence code is XP_027821574.1. Prostate Gene E synthetase-1 in NCBI has a gene ID of 9536 and an amino acid reference sequence code of NP_004869.1. Find the amino acids and their corresponding codons that cannot be expressed in E. coli, and then optimize the cyclooxygenase-1 and prostaglandin E synthase-1 genes to replace the codons that cannot be expressed in E. coli to ensure that the optimized genes can Expressed and active in E. coli. The optimized gene sequences of cyclooxygenase-1 and prostaglandin E synthase-1 are shown in SEQ ID NO.1 and SEQ ID NO.2.
[0041] At the two ends of the optimized cyclooxygenase-1 and prostaglandin E synthetase-1 gene sequences, the restriction site sequences of HindⅢ e...
Embodiment 2
[0043] Example 2 Preparation and Identification of Recombinant Expression Strains
[0044] 1. Preparation of Escherichia coli BL21(DE3) Competent Cells
[0045] (1) Strain activation: Escherichia coli BL21(DE3) stored in an ultra-low temperature refrigerator at -180°C was inoculated into 5mL LB liquid medium at an inoculation ratio of 1:50, and the medium was placed in a constant temperature shaker at 37°C , adjust 160rpm / min shaking culture overnight (12-16 hours), to OD 600 = about 0.4.
[0046] (2) Strain expansion: Take 100 μl of the strain activated in the previous step, add it to 5 ml of sterilized LB liquid medium, and shake and cultivate in a constant temperature shaker at 37° C. and 160 rpm / min for 2 hours.
[0047] (3) Use a pipette gun to take 1ml of the bacterial solution amplified in the previous step, dispense it into 1.5ml EP tubes, cool in an ice bath for 30min, and collect the bacterial cells at 4°C and 4000rpm for 10min.
[0048] (4) Discard the supernatan...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com