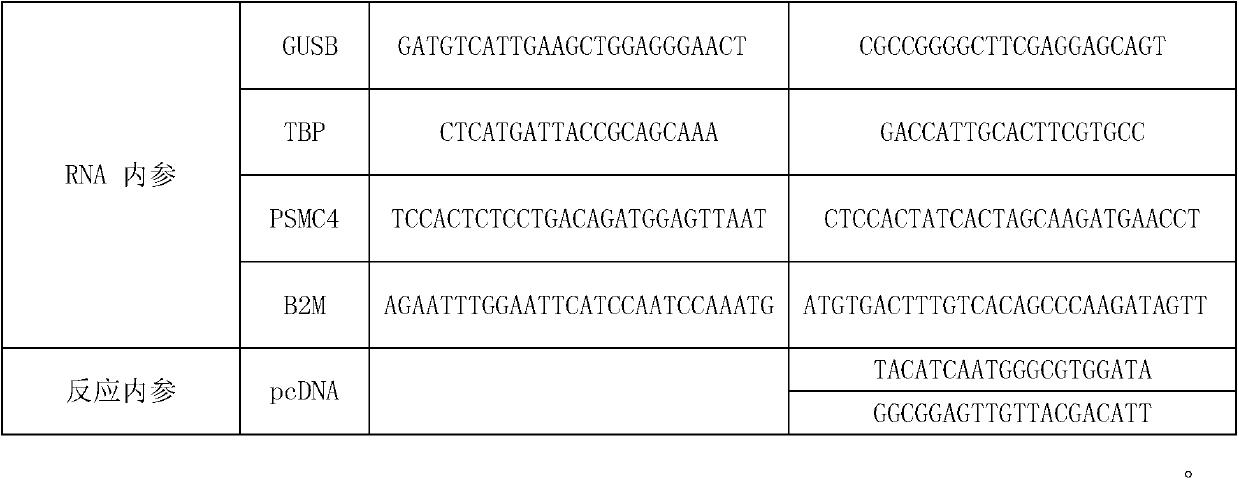
Patents
Literature
91results about How to "Solve easy pollution" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
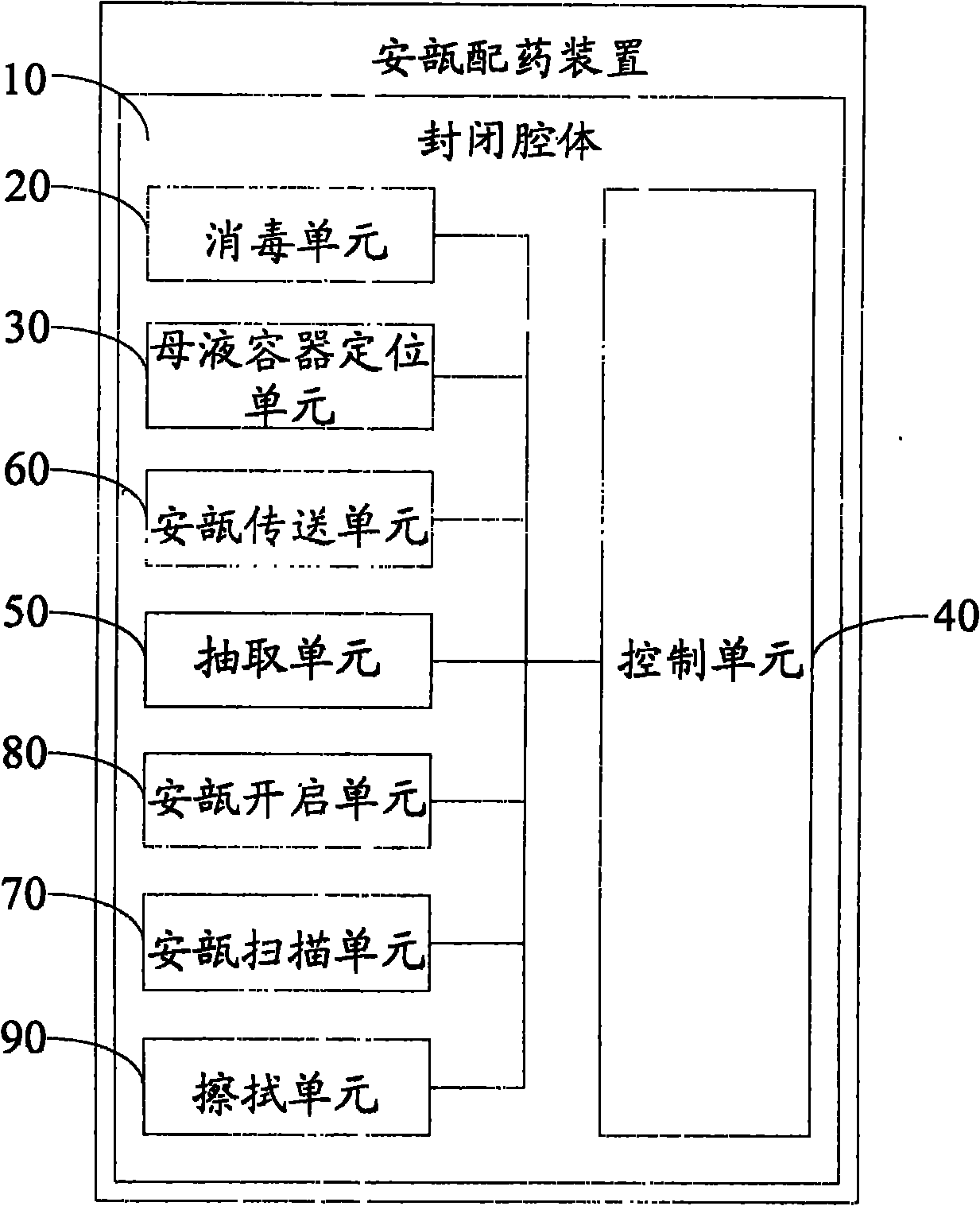
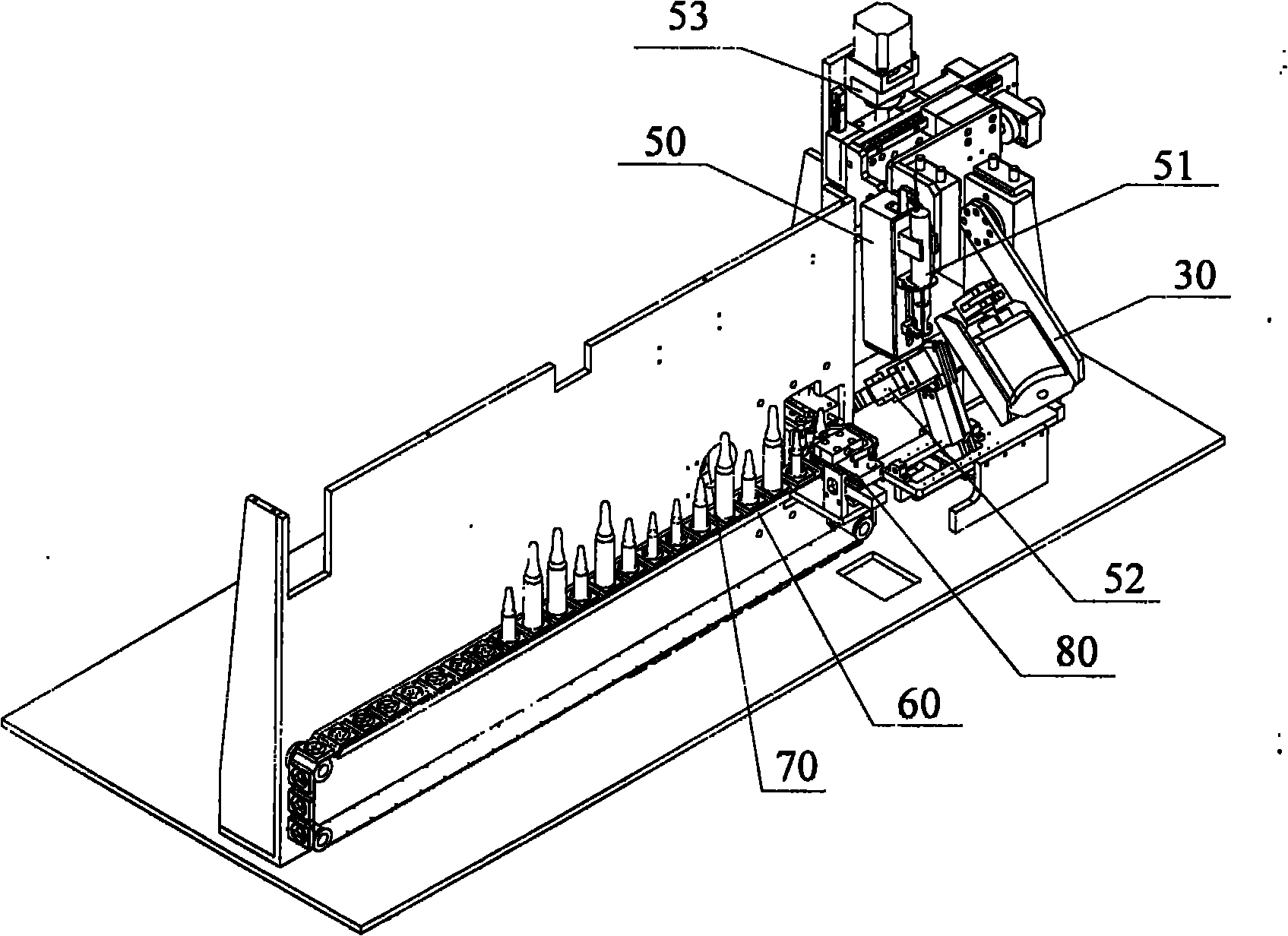
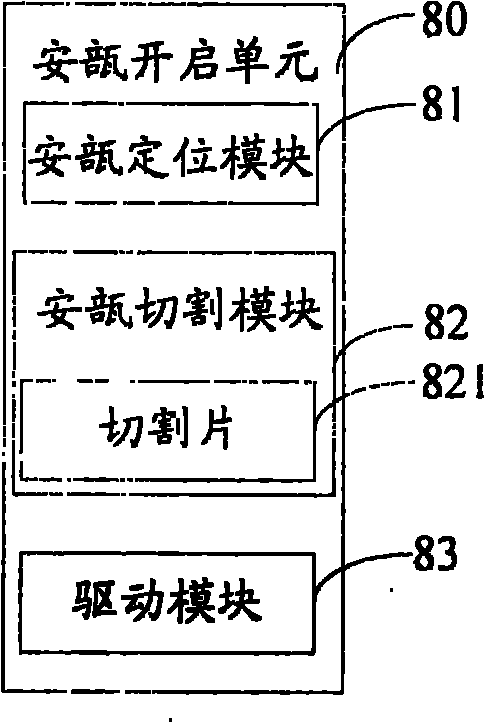
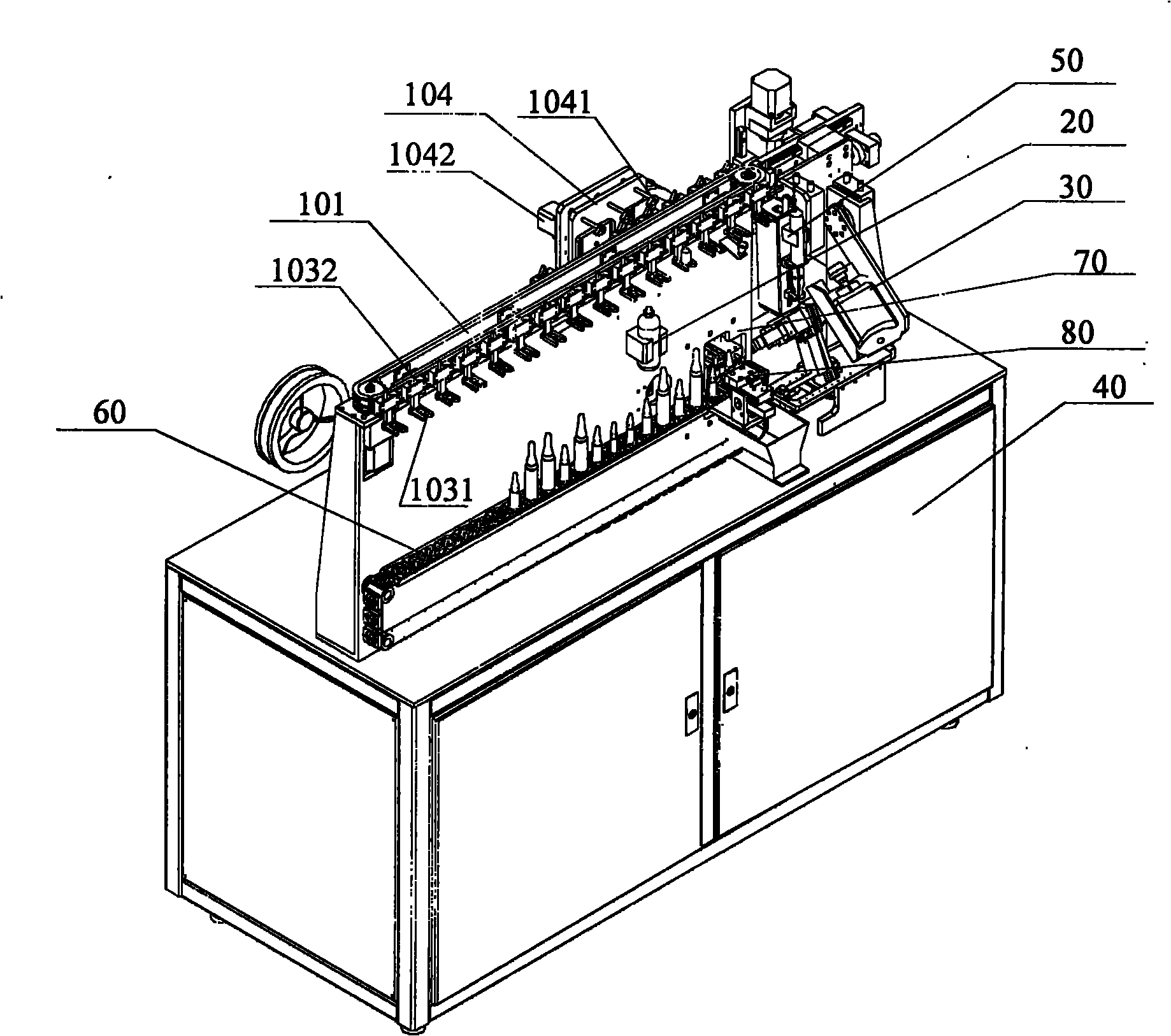
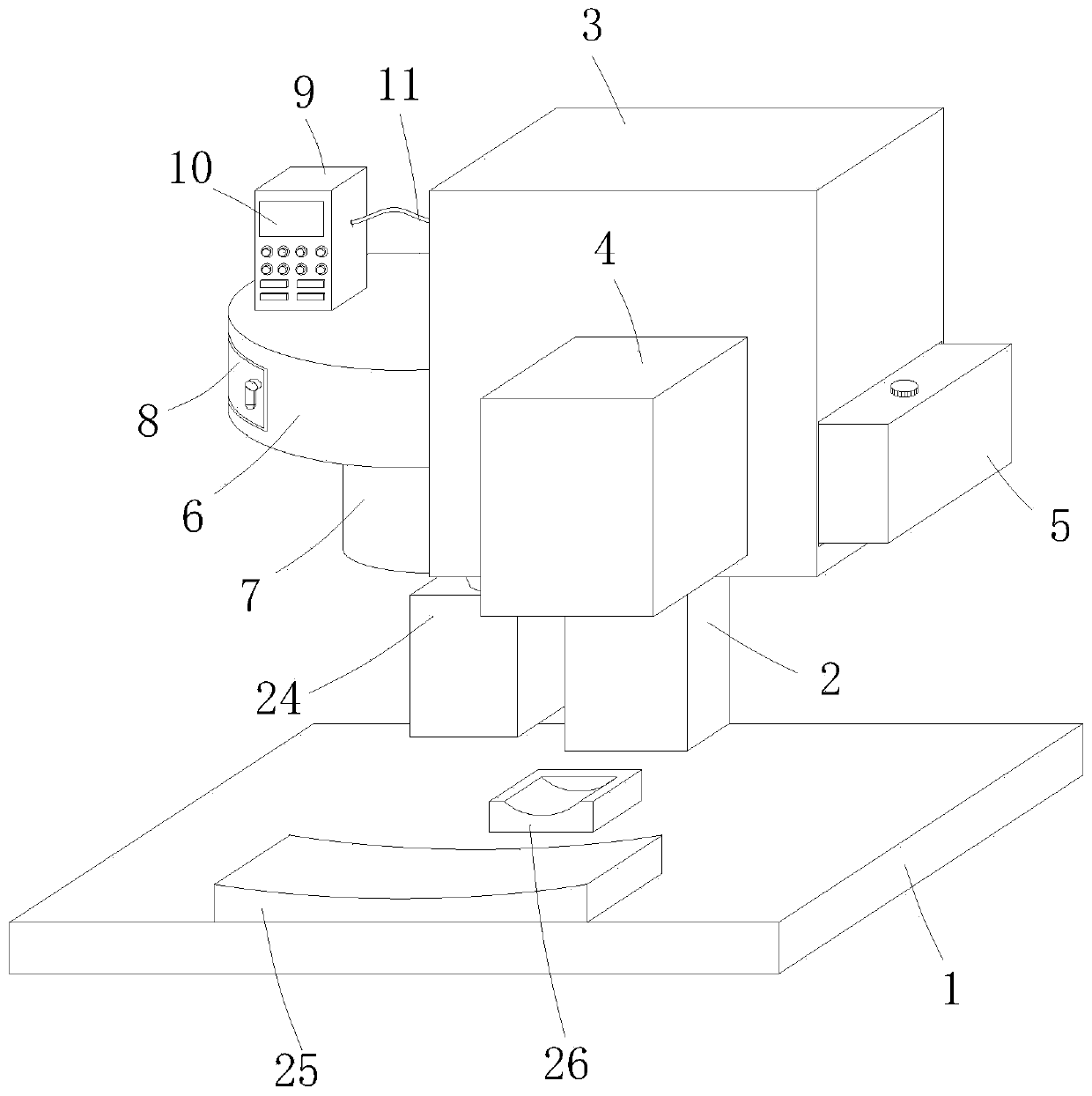
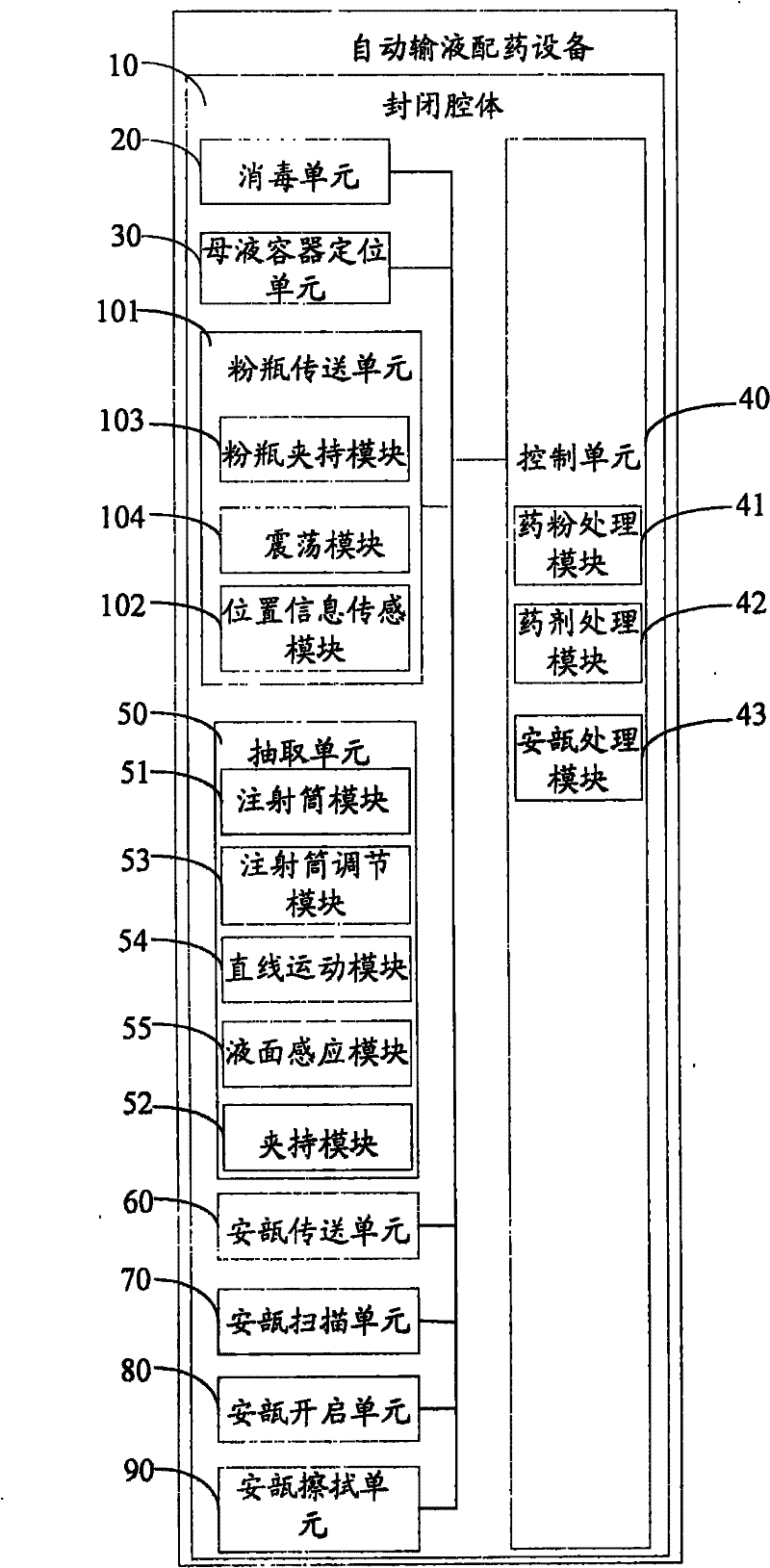
Ampule and powder bottle transfusion medicine dispensing devices and transfusion medicine dispensing method
ActiveCN101780010ASolve easy pollutionReduce riskPharmaceutical containersPharmaceutical product form changeBottleTransfusion medicine
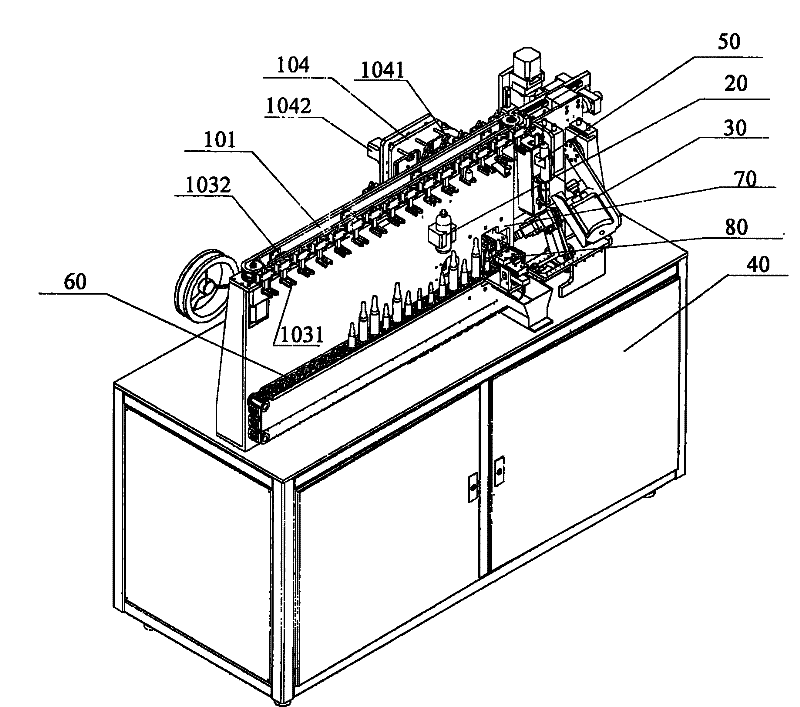
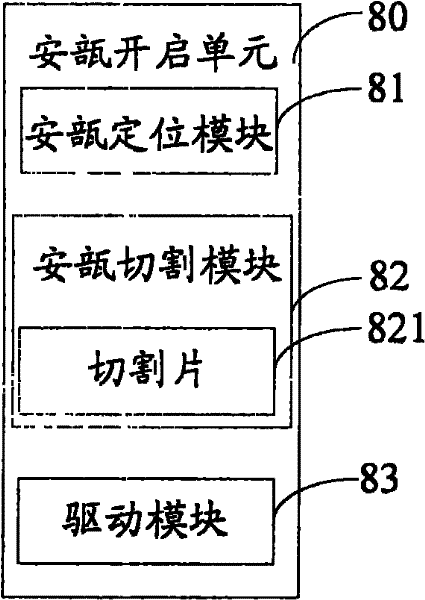
The invention relates to the technical field of transfusion medicine dispensation and provides an ampule transfusion medicine dispensing device. The ampule transfusion medicine dispensing device can automatically start an ampule and pump medicine liquid in the ampule to be mixed with mother liquid in a mother liquid container. The invention also provides a powder bottle transfusion medicine dispensing device which can automatically mix medicine powder or liquid in a powder bottle with the mother liquid in the mother liquid container. The invention also provides an automatic transfusion medicine dispensing method. By using the device or method in the invention, the liquid in the ampule and the medicine powder or liquid in the powder bottle are operated to be mixed with the mother liquid, the problem of easy pollution of the medicine liquid at present is solved, the medicine dispensing risk and the doctor and patient complication are lowered, the labor is saved, and the nursing efficiency and the nursing quality are improved.
Owner:山东卫邦智能机器人有限公司
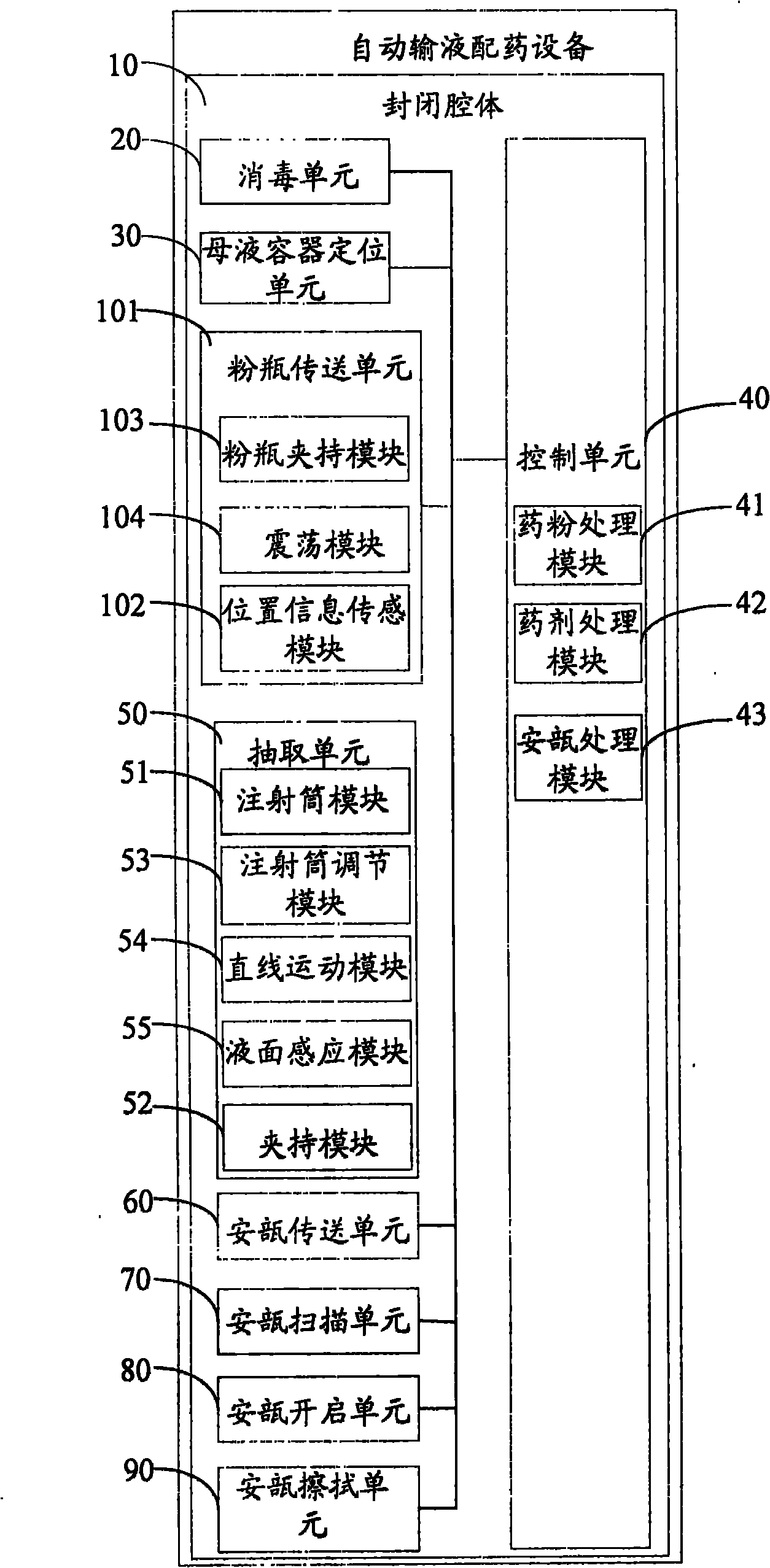
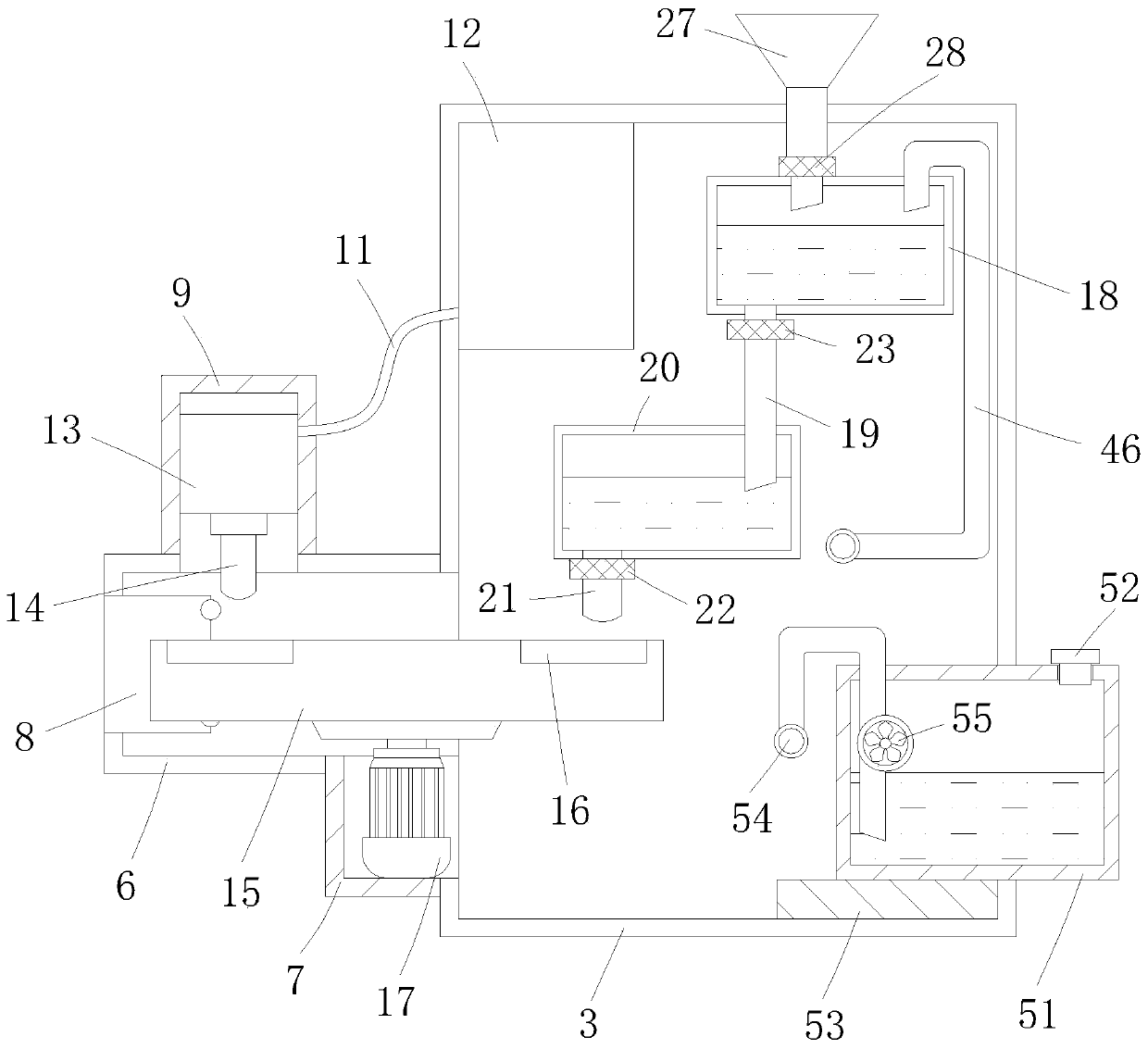
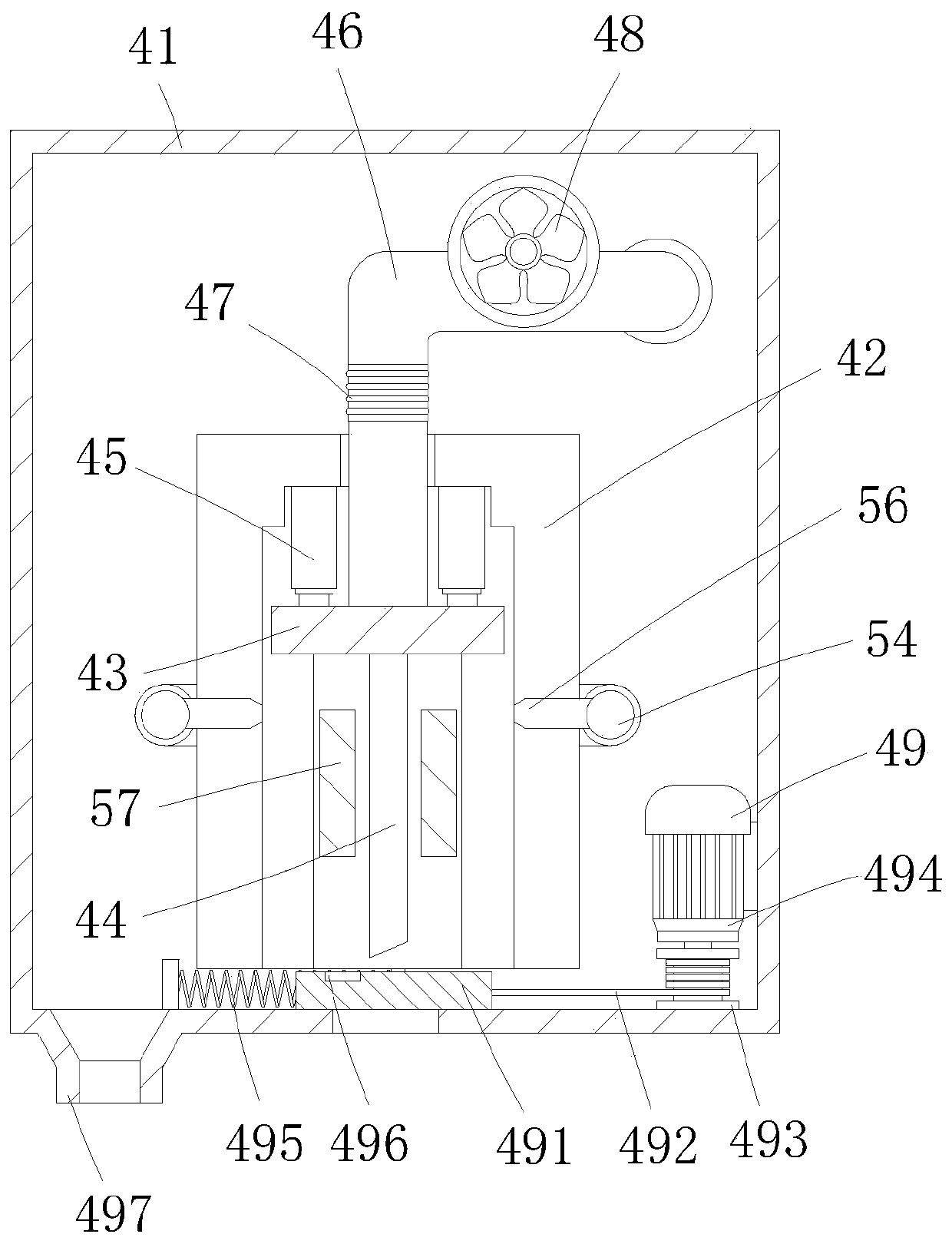
Automatic transfusion medicine dispensing equipment and automatic transfusion medicine dispensing method
ActiveCN101804225ASolve easy pollutionReduce riskInfusion devicesPharmaceutical product form changeBottleTransfusion medicine
The invention provides automatic transfusion medicine dispensing equipment, which belongs to the technical field of transfusion medicine dispensation. The automatic transfusion medicine dispensing equipment can automatically extract liquid in an ampoule, medicine powder or water aqua in a powder bottle, and mother liquid in a mother liquid container to be mixed. The invention also provides an automatic transfusion medicine dispensing method. The equipment and the method in the invention can be used for realizing the effect of operating the liquid in the ampoule and the medicine powder or the water aqua in the powder bottle by a machine and mixing the liquid in the ampoule and the medicine powder or the water aqua in the powder bottle with the mother liquid, the problem of easy pollution of the medicine liquid is solved, the medicine dispensing risk is lowered, the disputes between doctors and patients can be reduced, and in addition, the invention saves labor and improves the nursing efficiency and quality.
Owner:山东卫邦智能机器人有限公司
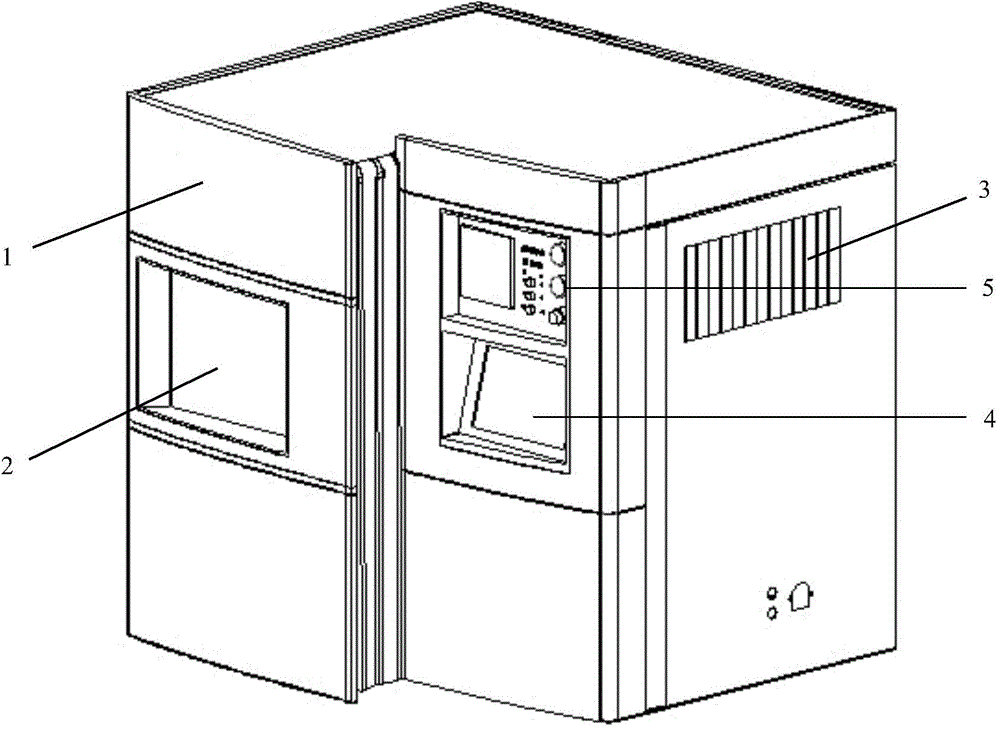
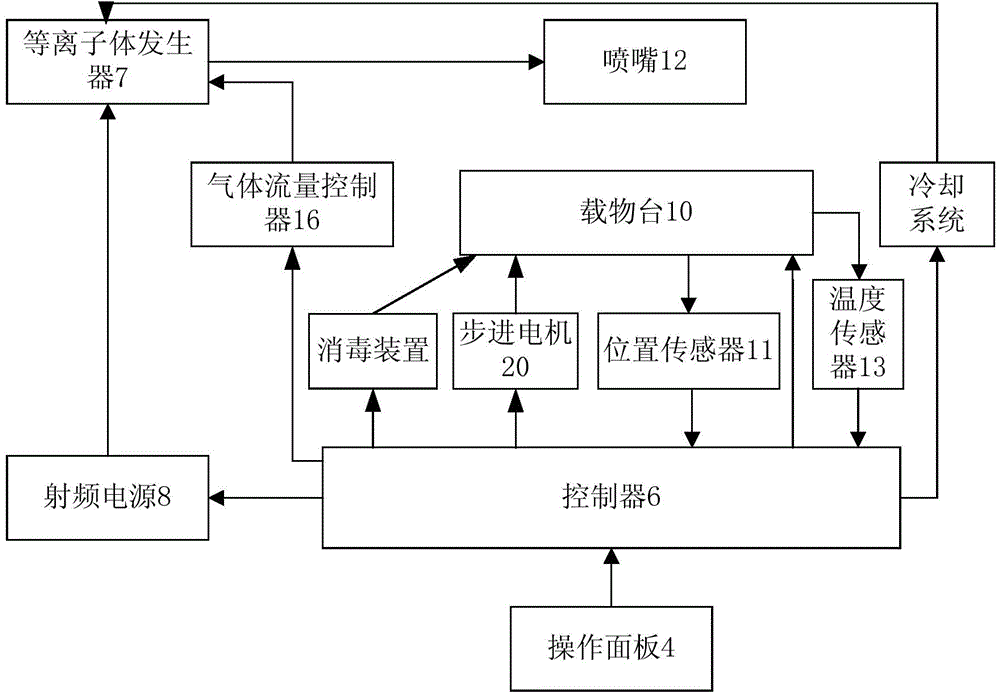
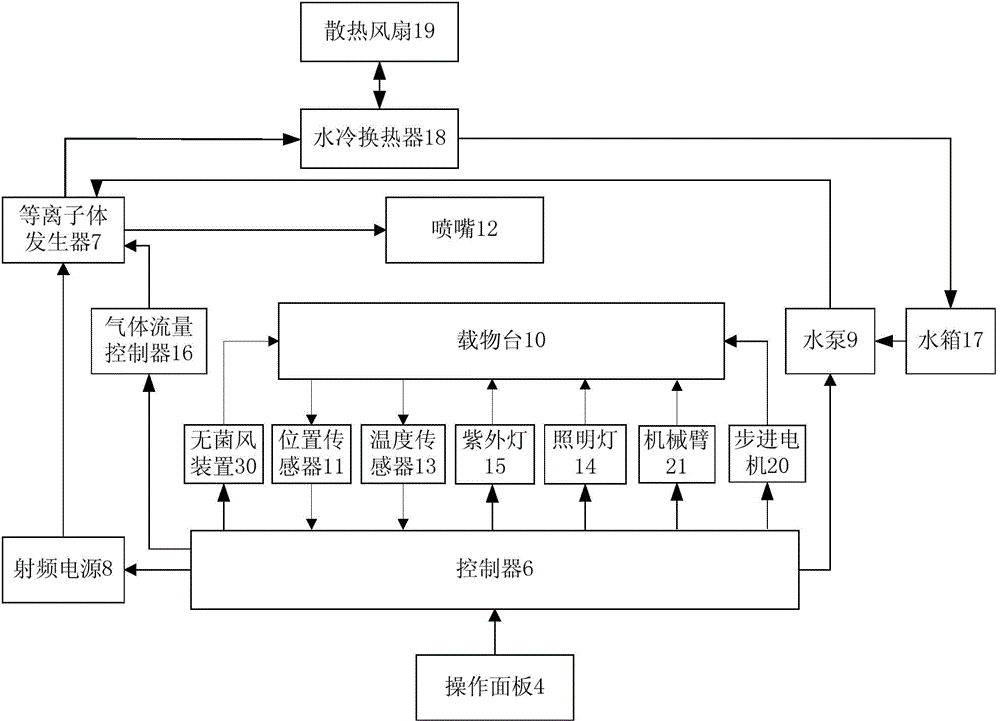
Novel normal-pressure room-temperature plasma induced mutation breeding device
ActiveCN103981091ASmooth dischargeEnables continuous automated processingBioreactor/fermenter combinationsBiological substance pretreatmentsInduced mutationControl system
The invention relates to a novel normal-pressure room-temperature plasma induced mutation breeding device. The novel normal-pressure room-temperature plasma induced mutation breeding device comprises a sample processing system, a cooling system, a control system and a detection system; the sample processing system comprises a clean working chamber without bioactive contaminants; a step motor and an object stage are arranged in the clean working chamber; the object stage is arranged on the step motor; at least one sterilizing device mounting position is reserved in the inner cavity or on the wall of the clean working chamber; the detection system comprises a gas flow controller, a temperature sensor and a position sensor; and the control system comprises an operating panel and a controller, and the controller is used for controlling the step motor to realize rising and falling or horizontal rotation of the object stage so as to automatically process a plurality of samples. The novel normal-pressure room-temperature plasma induced mutation breeding device is capable of realizing the functions of continuous automatic processing, automatic sterilization, automatic monitoring and control on the samples, and also capable of completing the plasma induced mutation breeding in the normal pressure and room temperature environment at higher efficiency.
Owner:WUXI TMAXTREE BIOTECHNOLOGY CO LTD
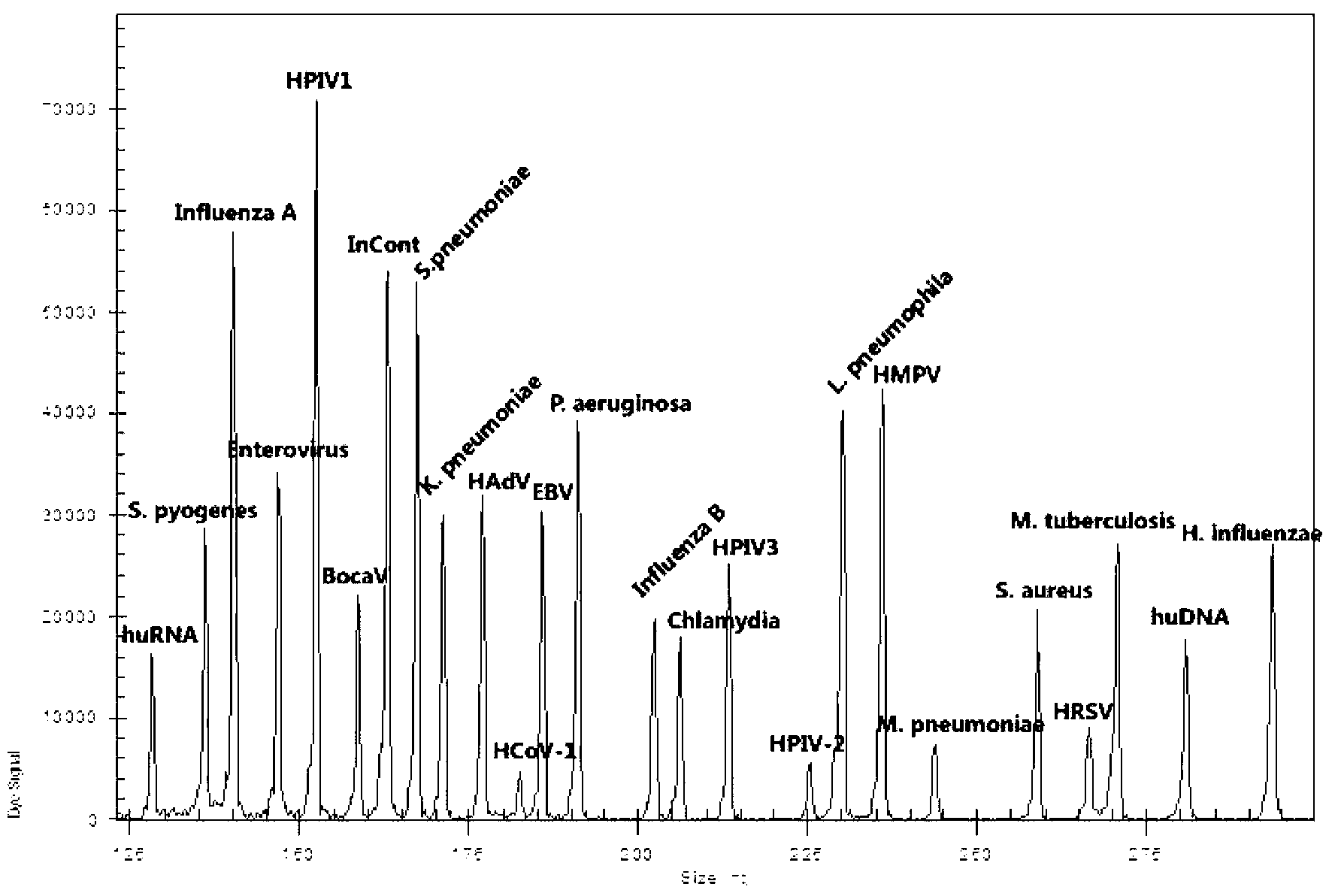
Method for simultaneously detecting twelve kinds of common respiratory viruses
InactiveCN104342503ASave production costSave testing costMicrobiological testing/measurementMicroorganism based processesMultiplexInfluenza virus C
The invention discloses a method for simultaneously detecting twelve kinds of common respiratory viruses. According to the method, primers and probes are designed according to gene conservative areas of the twelve kinds of common respiratory viruses, namely influenza A virus, influenza B virus, influenza C virus, parainfluenza virus type 1, parainfluenza virus type 2, parainfluenza virus type 3, rhinovirus, Bocavirus, adenovirus, coronavirus, metapneumovirus and respiratory syncytial virus, nucleic acid fragments of samples to be measured are extracted for amplifying, and finally, the samples are separated by using a capillary electrophoresis method. The method disclosed by the invention has the advantages of low required sample size, high sensitivity and accuracy, good specificity and low cost; the defects that the conventional single tube multiplex fluorescence PCR (Polymerase Chain Reaction) detection primers are difficult to design, and multicolor fluorescence mutually intervenes and is not easy to part are overcome, the defects that a chip detection method is tedious in operation, high in detection cost and the like are also overcome, and a new method is provided for screening the respiratory viruses.
Owner:FUJIAN INT TRAVEL HEALTH CARE CENT +1
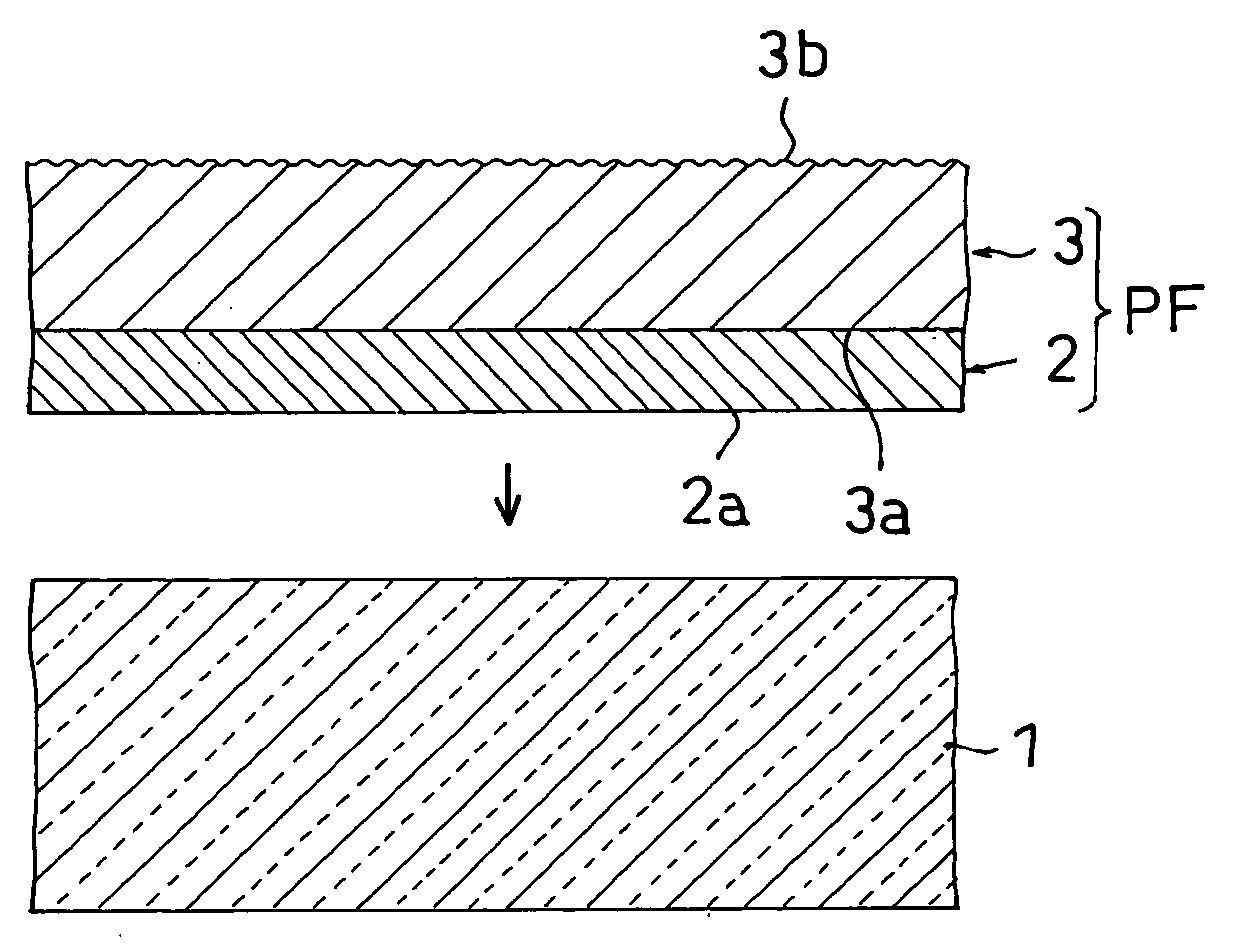
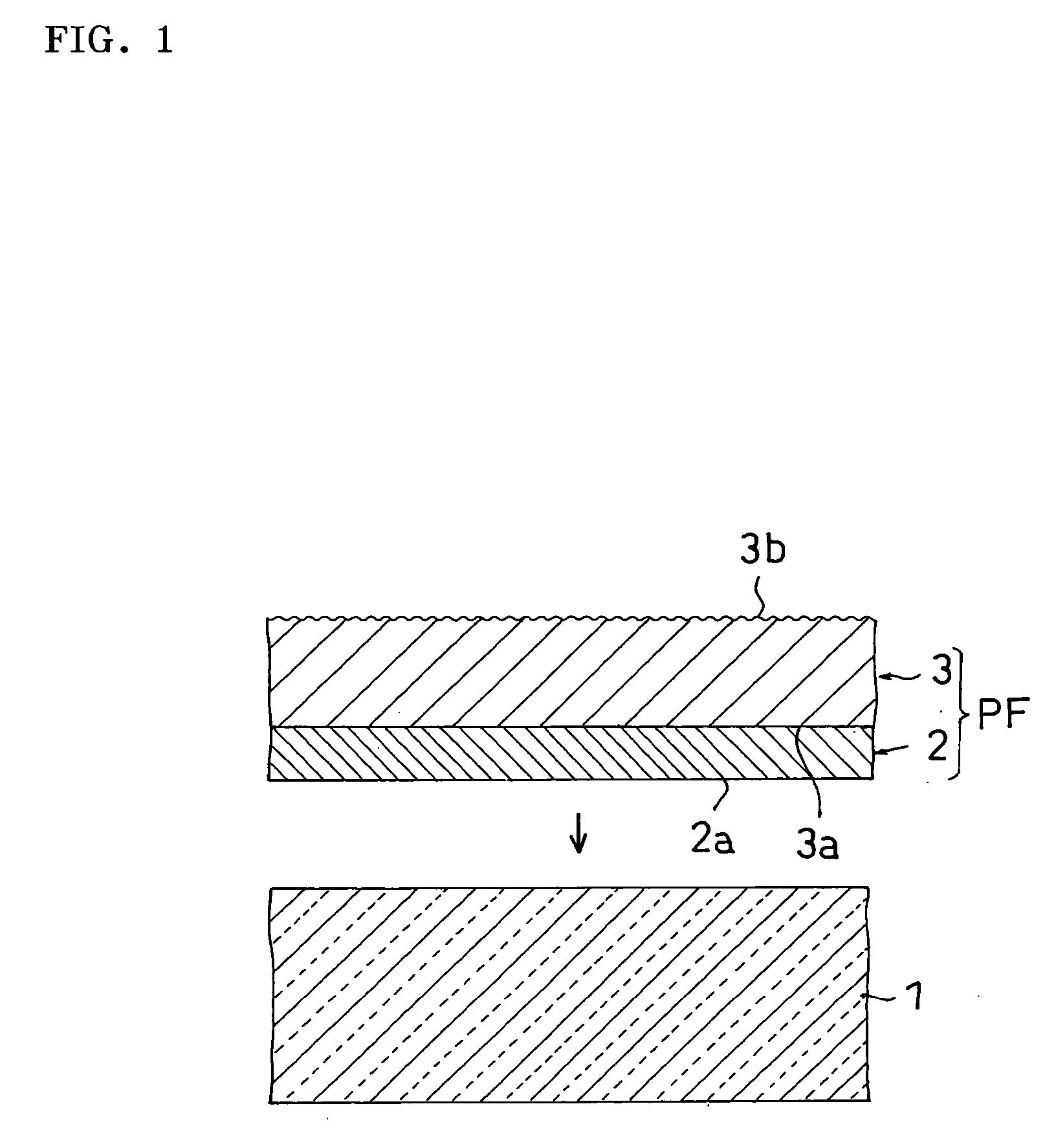
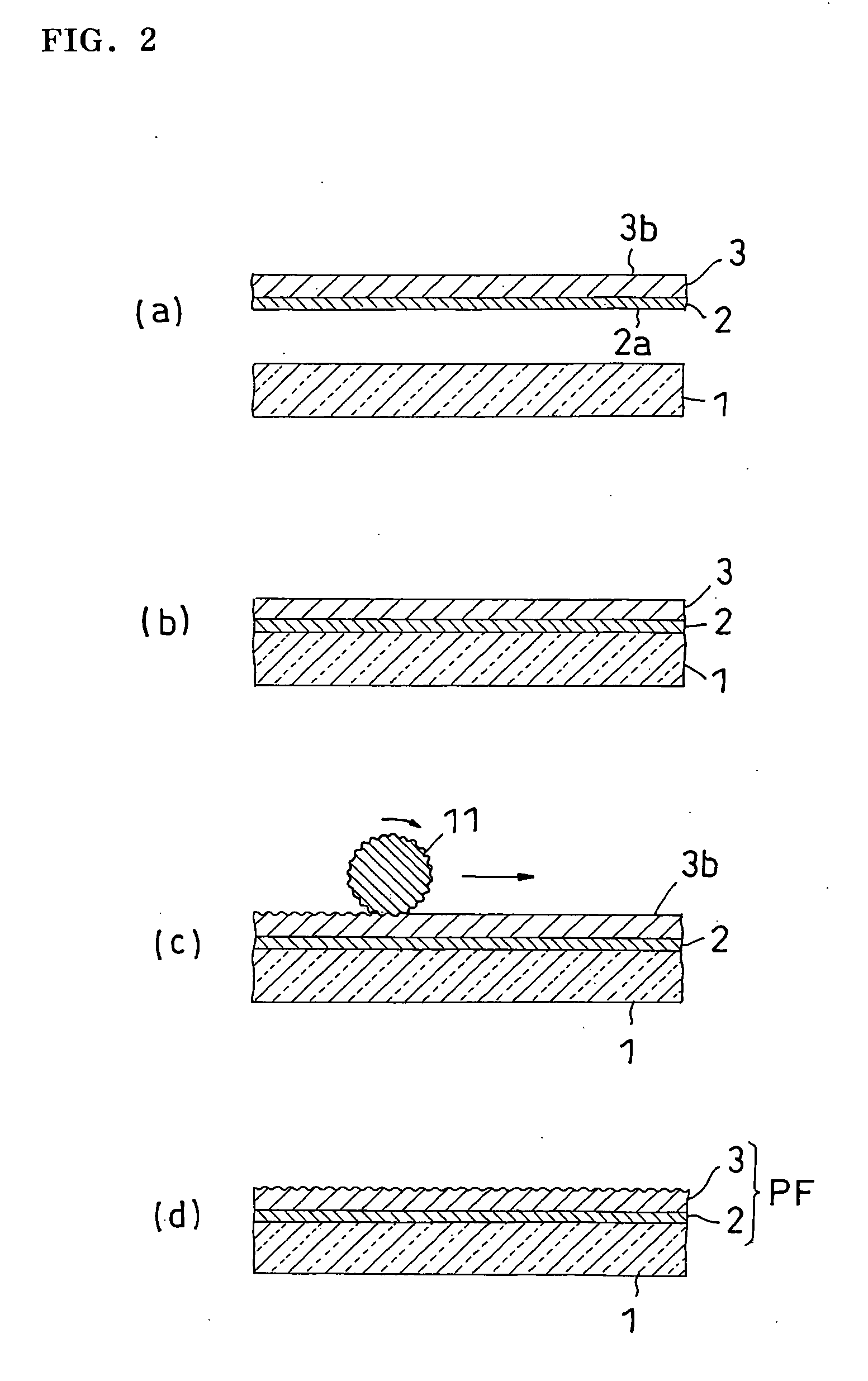
Film for protecting mother glass for flat panel display and use thereof
InactiveUS20050253276A1Smooth transferGood effectFilm/foil adhesivesSemiconductor/solid-state device detailsTectorial membraneDisplay device
Owner:NITTO DENKO CORP
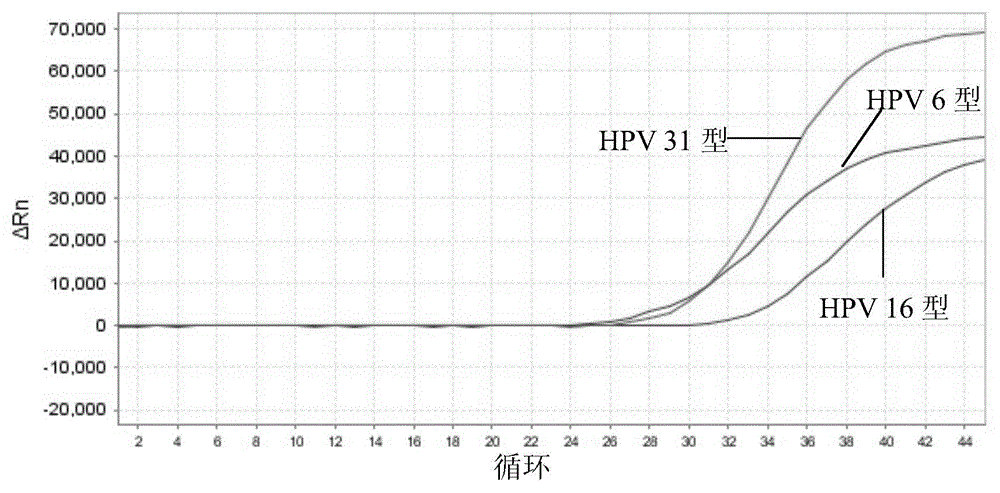
Nucleic acid detection kit for human papilloma virus, use method and application thereof
ActiveCN105506173AAvoid false negativesIncrease throughputMicrobiological testing/measurementMicroorganism based processesLower riskFluorescence
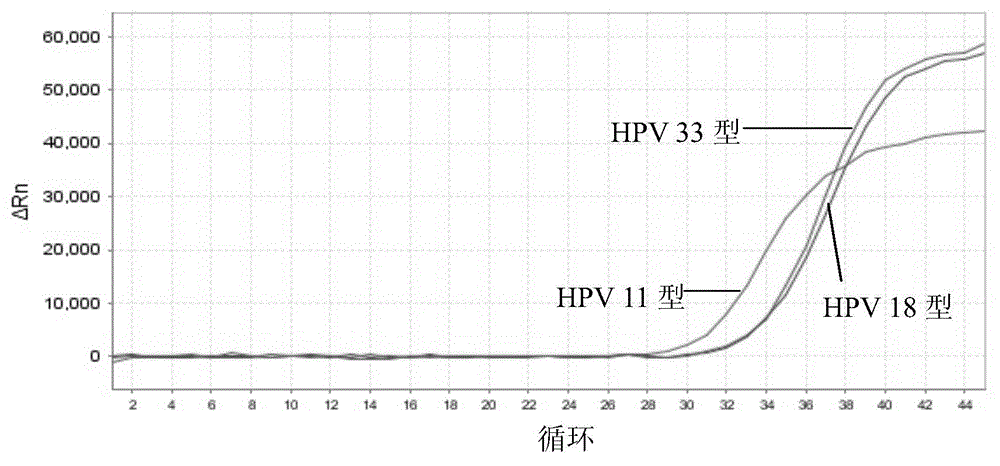
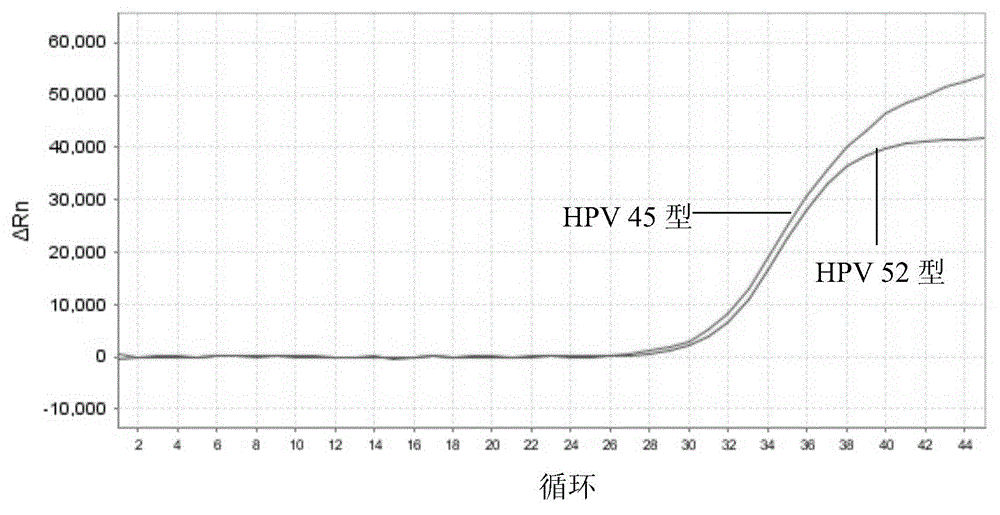
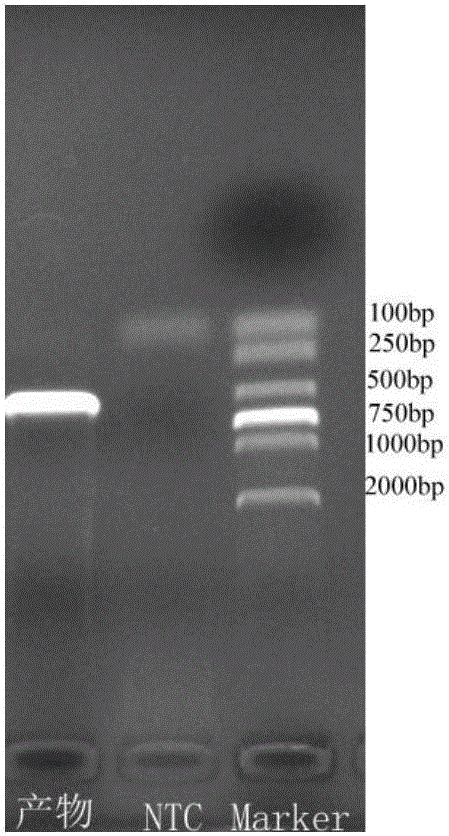
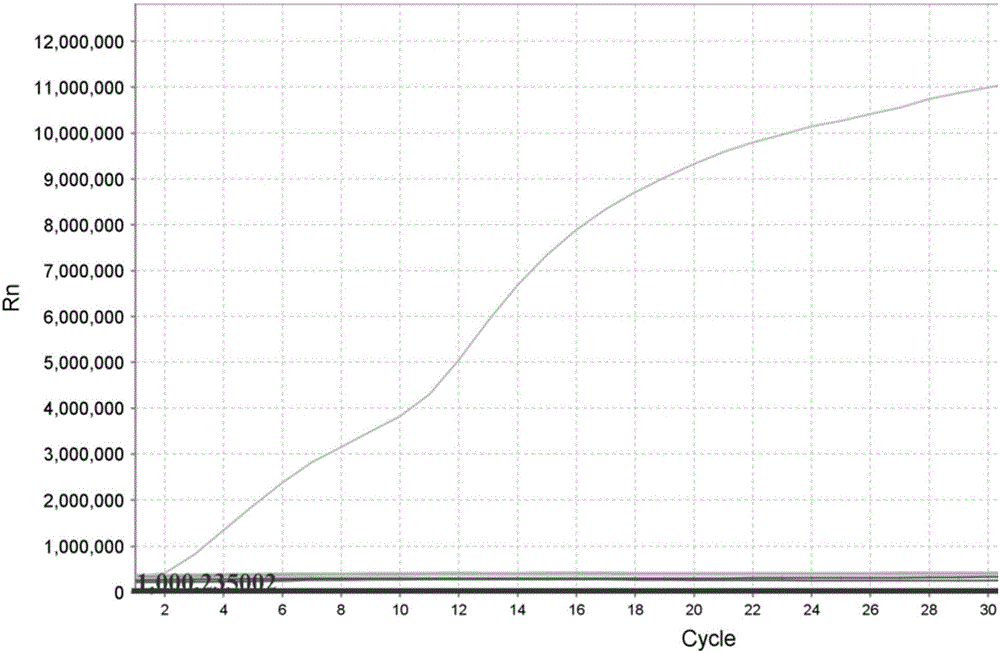
The invention discloses a nucleic acid detection kit for human papilloma virus, a use method and an application thereof. The kit includes a nucleic acid amplification reagent comprising a primer pair and a probe corresponding to the primer pair. The use method of the kit includes the following steps: 1) extracting nucleic acids from a sample; 2) preparing the reagent; 3) performing PCR amplification; and 4) performing fluorescent detection to a PCR amplification reaction product at 65-72 DEG C, and determining infection type of the HPV according to the changes on the Ct value of a target amplification curve and the Ct value of an internal standard amplification curve. The kit can be used for detecting low-risk and high-risk human papilloma virus and for typing the human papilloma virus. The kit can be used for calculating relative viral load of the HPV, can avoid pollution, can increase treatment throughput of samples, and can avoid missing detection.
Owner:SUZHOU SYM BIO LIFESCI CO LTD
Nucleic acid for detecting Zika virus, real-time fluorescence RPA kit and method
ActiveCN106367533AEasy to useEasy to detectMicrobiological testing/measurementDNA/RNA fragmentationZika virusFluorescence
The invention discloses nucleic acid for detecting Zika virus, a real-time fluorescence RPA kit and a detection method thereof. The real-time fluorescence RPA kit is convenient to use, the quantity of reagents adopted is small, the cost is low, the compatibility of required instruments is high, reaction can be performed on a real-time fluorescence PCR instrument and instruments with a fluorescent trapping function, and the greatest advantage of the kit lines in performing isothermal amplication and being capable of detecting fluorescence signals in real time within 10 to 30 min. The detection method greatly simplifies the operation process, reduces steps of repetitive operation, saves time, reduces labor force consumed by repetitive operation, and effectively lowers the cost, and a detection result shows that the method is high in specificity and sensitivity, and rapid and accurate screening of Zika virus is realized.
Owner:淮安市疾病预防控制中心
Kit for synchronously detecting twenty-three meningitis pathogens and detection method of kit
ActiveCN103074448AEnsuring Quality JudgmentsStrong specificityMicrobiological testing/measurementMicroorganism based processesPositive controlReverse transcriptase
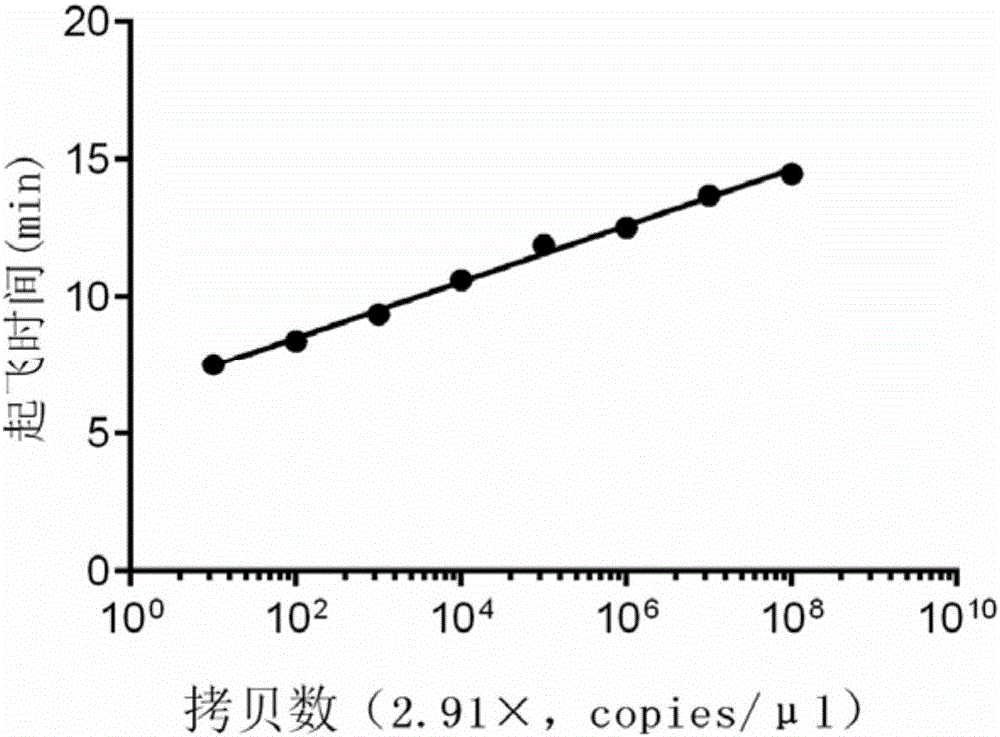
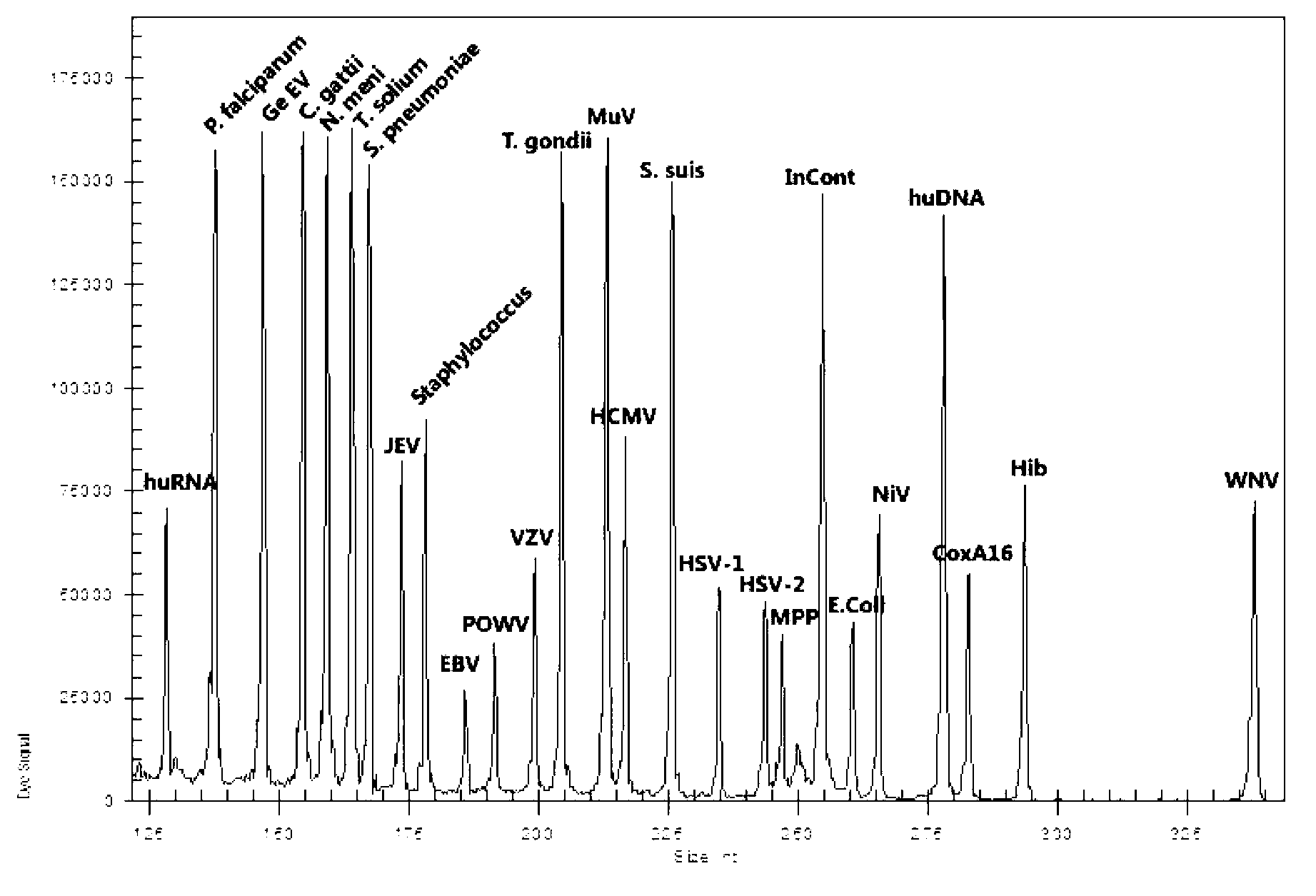
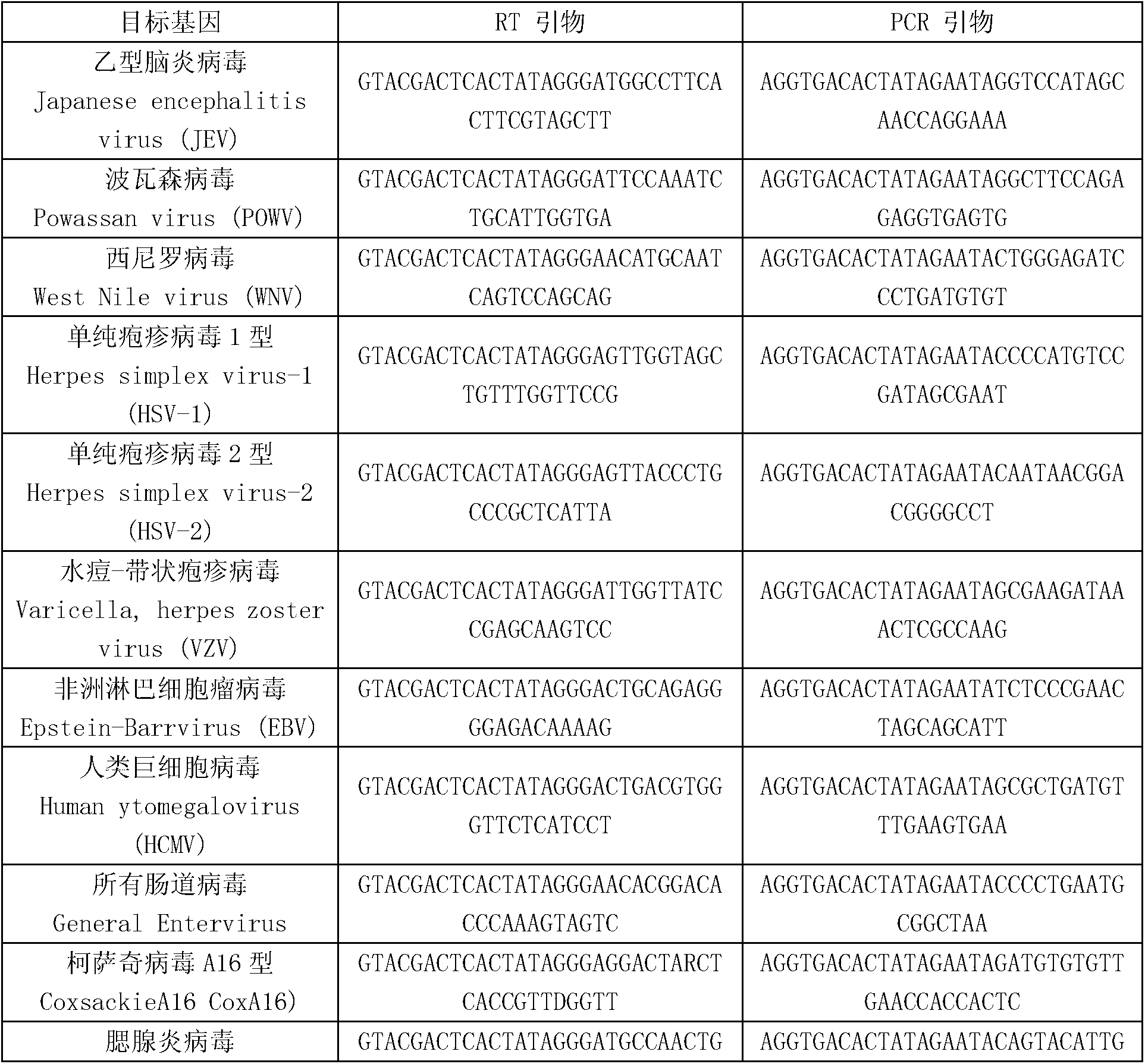
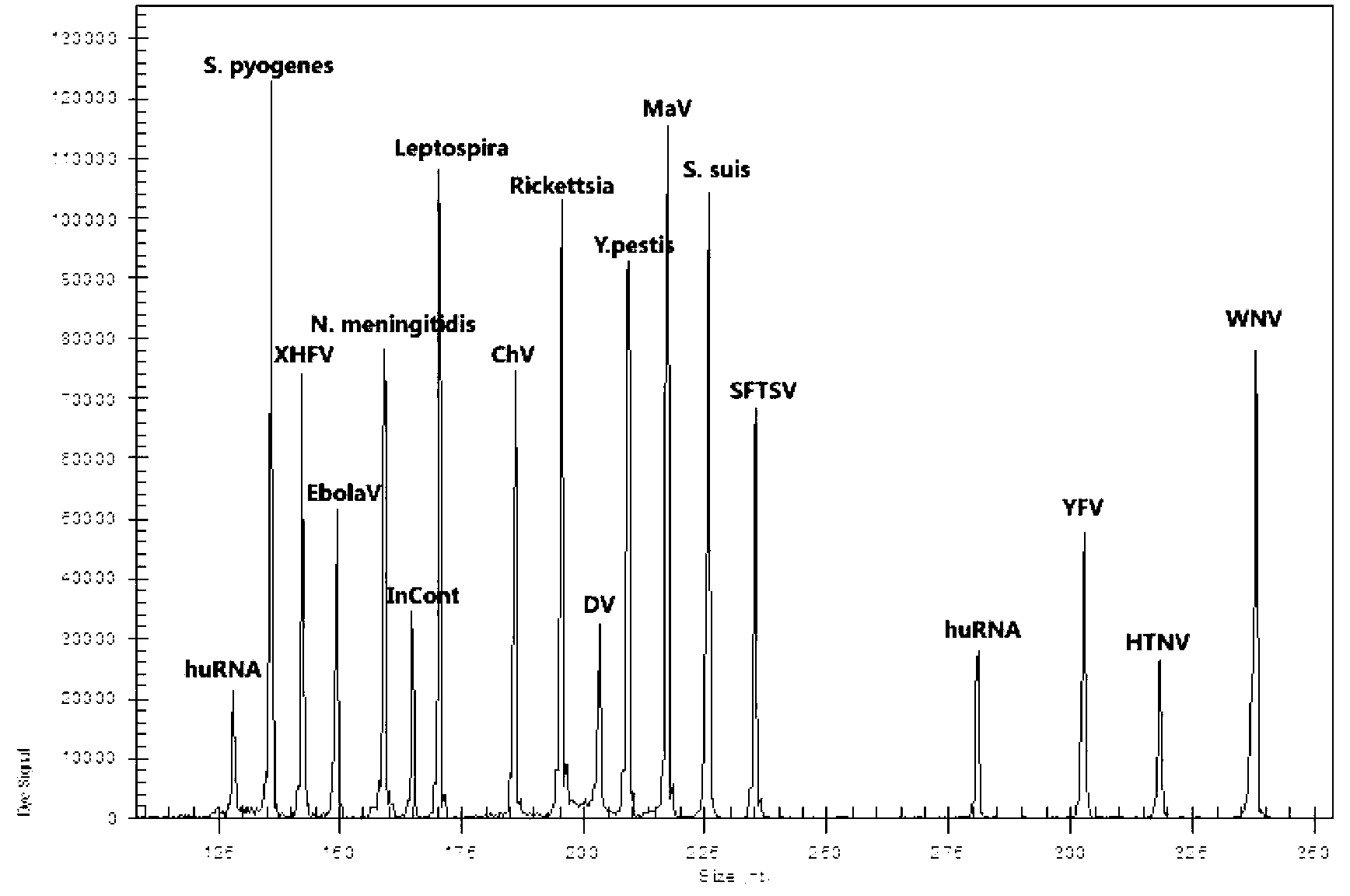
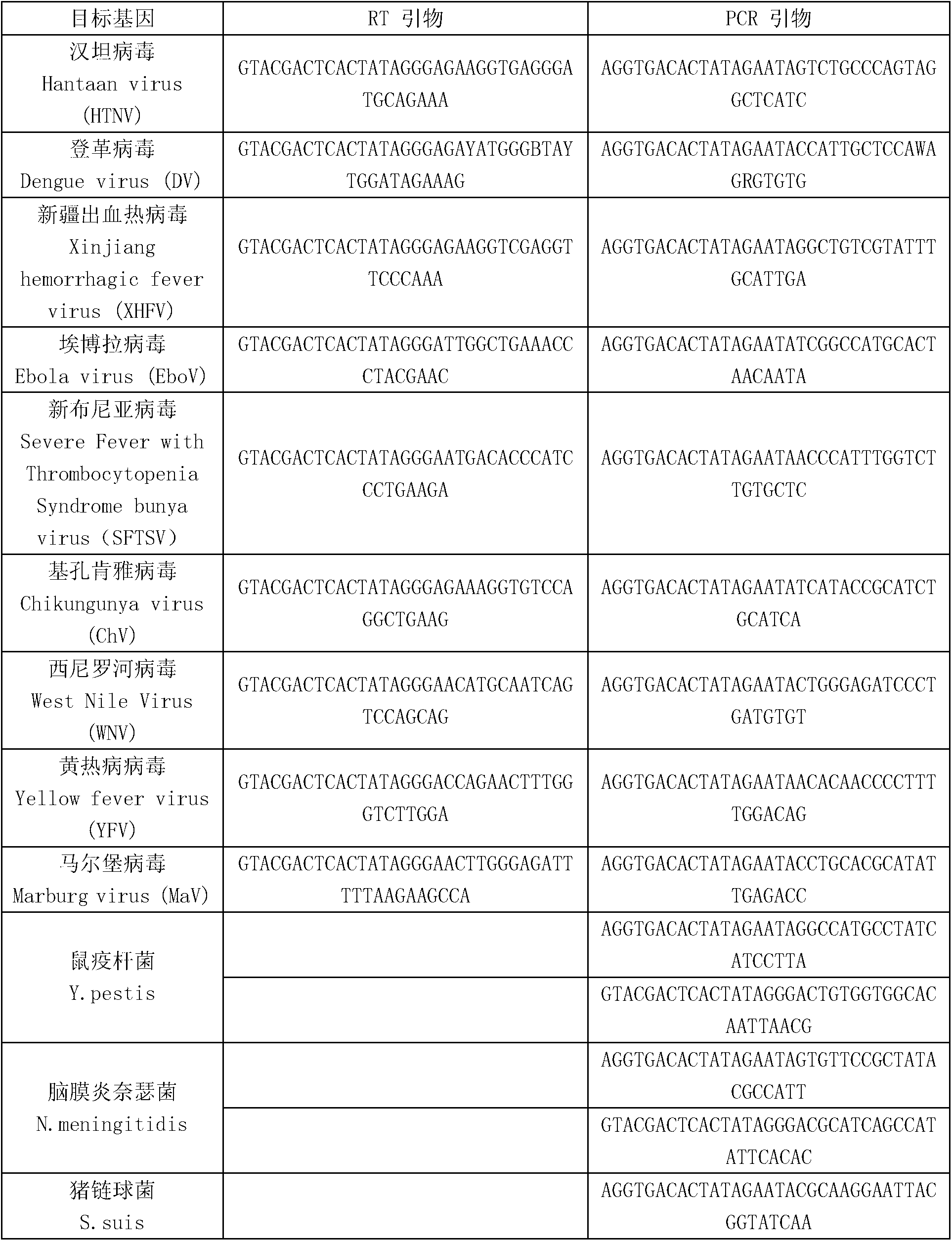
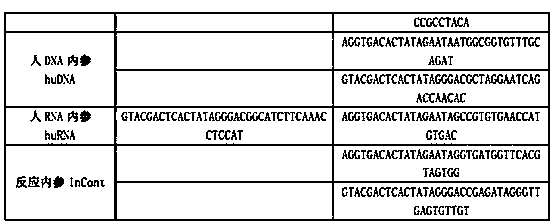
The invention discloses a kit for synchronously detecting twenty-three meningitis pathogens and a detection method of the kit. The kit comprises DEPC (diethylpyrocarbonate) water, a 5*RT (reverse transcription) buffer, a reverse transcription primer, a reverse transcriptase, an X solution, a 10*PCR (polymerase chain reaction) buffer, a PCR primer, a 25mM magnesium chloride solution, a DNA (deoxyribonucleic acid) polymerase and a positive control, and is characterized in that the reverse transcription primer comprises RT amplification primers of the twelve meningitis pathogens and a human RNA (ribonucleic acid) internal reference, and has a gene sequence shown as SEQ ID NO. 1-13 (sequence identifier number 1-13), and the PCR primer comprises forward and reverse PCR amplification primers of the rest eleven meningitis pathogens, a human DNA internal reference and a reaction internal reference, and PCR amplification primers of the twelve meningitis pathogens and the human RNA internal reference, and has a gene sequence shown as SEQ ID NO. 14-52. The kit and the detection method have the advantages of high specificity, sensitivity, flux and reliability, low cost, and no false negative results.
Owner:NINGBO HEALTH GENE TECHNOLOGIES CO LTD
Kit for synchronously detecting twenty-two respiratory tract pathogens and detection method of kit
ActiveCN103074451AEnsuring Quality JudgmentsImprove detection efficiencyMicrobiological testing/measurementMicroorganism based processesPositive controlReverse transcriptase
The invention discloses a kit for synchronously detecting twenty-two respiratory tract pathogens and a detection method of the kit. The kit comprises DEPC (diethylpyrocarbonate) water, a 5*RT (reverse transcription) buffer, a reverse transcription primer, a reverse transcriptase, an X solution, a 10*PCR (polymerase chain reaction) buffer, a PCR primer, a 25mM magnesium chloride solution, a DNA (deoxyribonucleic acid) polymerase and a positive control, and is characterized in that the reverse transcription primer comprises RT amplification primers of the nine respiratory tract pathogens, and a reverse primer of a human RNA (ribonucleic acid) internal reference, and has a gene sequence shown as SEQ ID NO. 1-6 (sequence identifier number 1-6), and the PCR primer comprises forward and reverse PCR amplification primers of the rest thirteen respiratory tract pathogens, a forward and reverse amplification primer of a human DNA internal reference, a forward and reverse PCR amplification primer of a reaction internal reference, PCR amplification primers of the nine respiratory tract pathogens, and a PCR amplification primer of the human RNA internal reference, and has a gene sequence shown as SEQ ID NO. 7-44. The kit and the detection method have the advantages of high specificity, sensitivity, flux and reliability, low cost, and no false negative results.
Owner:NINGBO HEALTH GENE TECHNOLOGIES CO LTD
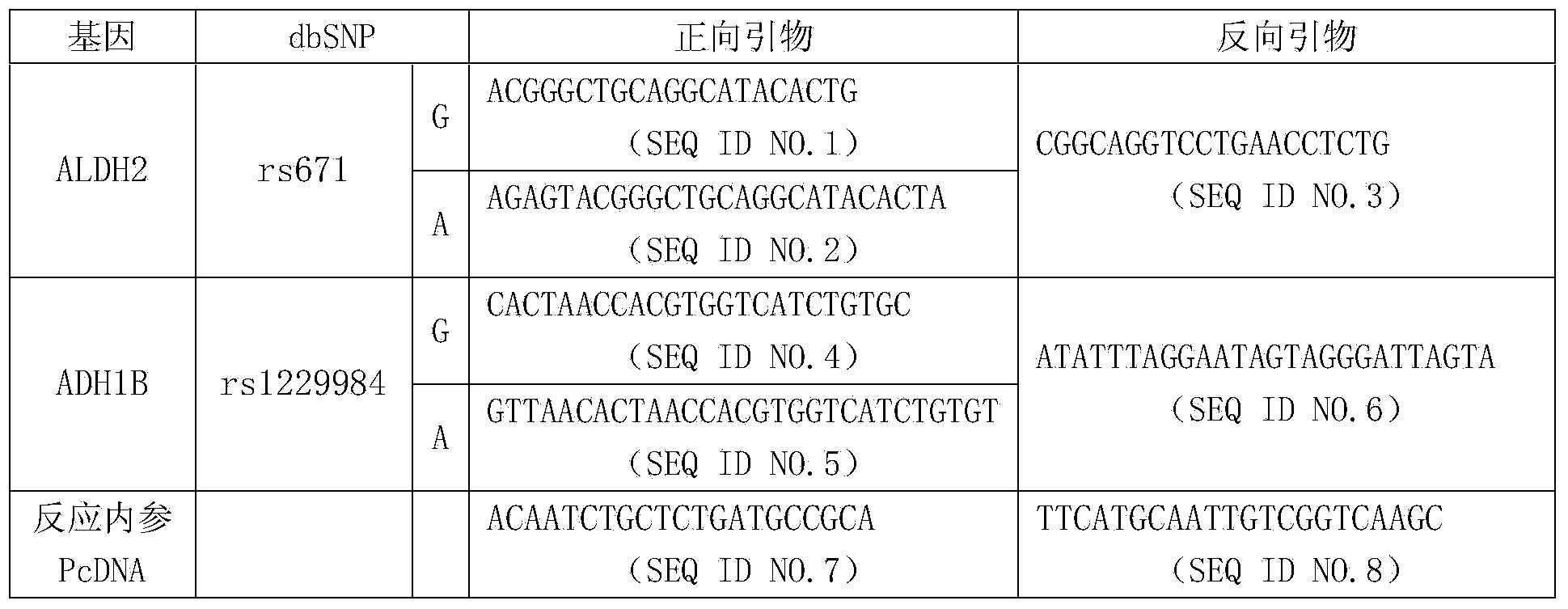
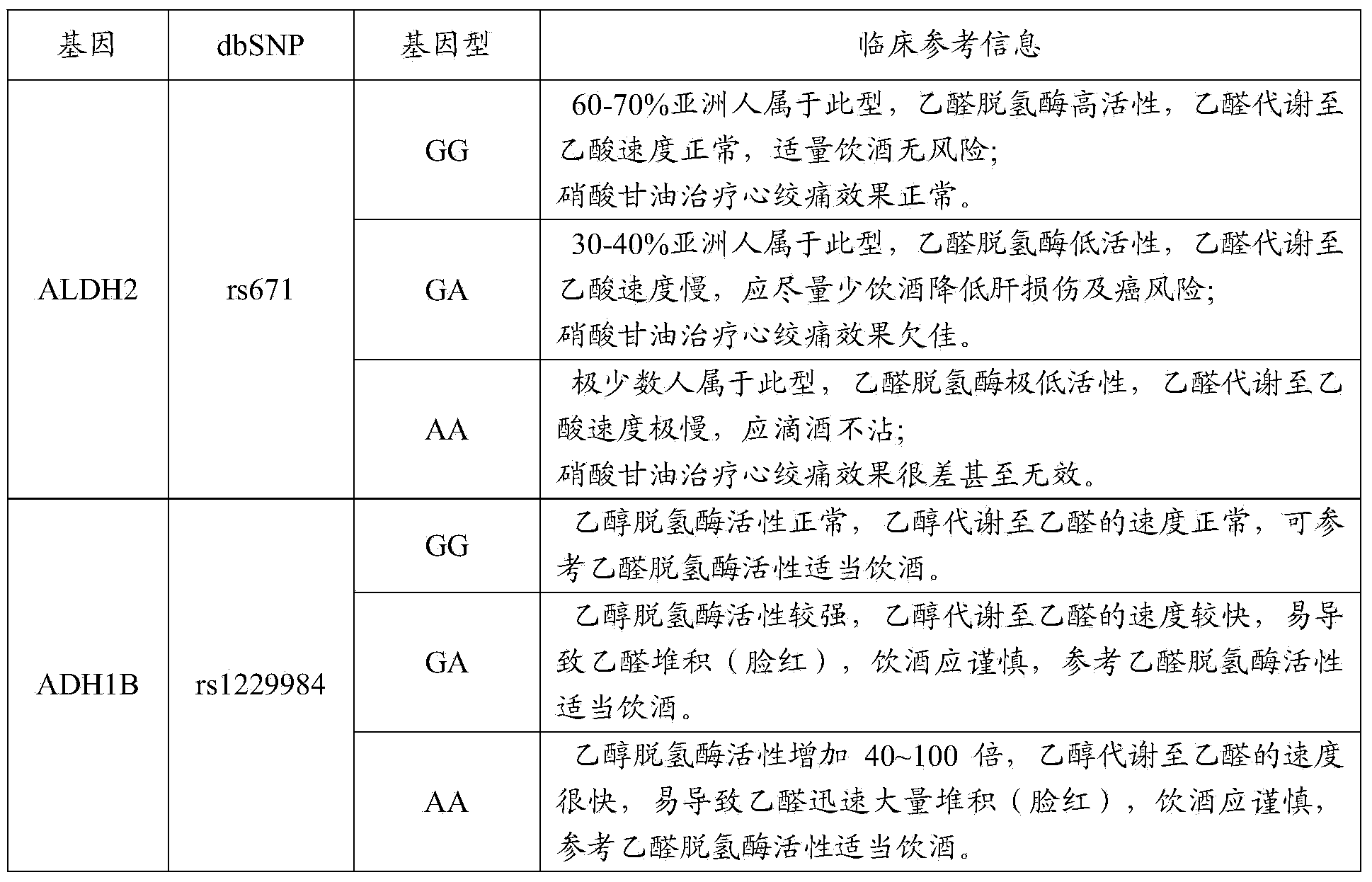
Primer composition for guiding nitroglycerin medication and healthy drinking, multiple gene detection kit and use method of kit
ActiveCN103849681ASave production costSave testing costMicrobiological testing/measurementCapillary electrophoresisGene type
The invention discloses a primer composition for guiding nitroglycerin medication and healthy drinking, a multiple gene detection kit and a use method of the kit, and the kit comprises the primer composition, a PCR buffer solution and a positive reference substance, and the PCR buffer solution comprises ultrapure water, an X solution, a 10*PCR (polymerase chain reaction) buffer solution, a PCR primer, a 25mM magnesium chloride solution and DNA polymerase, the primer composition comprise two forward and reverse amplification primers of different gene types on the 2 SNP sites of genes related to the nitroglycerin medication and healthy drinking and forward and reverse amplification primers capable of reflecting internal reference, the gene sequences of the primers are represented as SEQ ID NO.1-NO.8; the use method comprises the step of acquiring a sample and extracting nucleic acid, the step of performing the PCR reaction by using extracted nucleic acid as a template, and the final step of separating the sample through capillary electrophoresis. The primer composition has the advantages of being strong in specificity, high in accuracy, high in flux, strong in reliability, low in cost and free from false negative result.
Owner:NINGBO HEALTH GENE TECHNOLOGIES CO LTD
Deep treatment method of high-concentration organic wastewater
ActiveCN108358362AStable in natureEasy to recycleWater/sewage treatment by irradiationWater/sewage treatment by magnetic/electric fieldsHigh concentrationElectrolysis
The invention discloses a deep treatment method of high-concentration organic wastewater. The method comprises the following steps: step one, coagulating and precipitating; step two, carrying out ultraviolet-ozone-ultrasound combined reaction stage; step three, carrying out electrolysis treatment through three dimension electrodes; step four, recycling heavy metals; and step five, carrying out ultrafiltration-electrodialysis coupled desalinization. The deep treatment method of high-concentration organic wastewater is free of biological treatment processes, free of pretreatment, high in desalinization rate, simple to operate, high in efficiency, free of secondary pollution and low in cost, and is capable of completely removing organic matters, recycling the heavy metals and recycling the high-concentration organic wastewater.
Owner:江苏中丽新材料有限公司
Multi-gene detection kit for guiding administration of 5-fluorouracil and detection method of multi-gene detection kit
ActiveCN103074436AImprove detection efficiencyShorten the timeMicrobiological testing/measurementPositive controlPolymerase L
Owner:NINGBO HEALTH GENE TECHNOLOGIES CO LTD
Kit for synchronously detecting fifteen hemorrhagic fever pathogens and detection method of kit
ActiveCN103074452AMonitor reaction efficiencyEnsuring Quality JudgmentsMicrobiological testing/measurementMicroorganism based processesPositive controlReverse transcriptase
The invention discloses a kit for synchronously detecting fifteen hemorrhagic fever pathogens and a detection method of the kit. The kit comprises DEPC (diethylpyrocarbonate) water, a 5*RT (reverse transcription) buffer, a reverse transcription primer, a reverse transcriptase, an X solution, a 10*PCR (polymerase chain reaction) buffer, a PCR primer, a 25mM magnesium chloride solution, a DNA (deoxyribonucleic acid) polymerase and a positive control, and is characterized in that the reverse transcription primer comprises RT amplification primers of the nine hemorrhagic fever pathogens and a human RNA (ribonucleic acid) internal reference, and has a gene sequence shown as SEQ ID NO. 1-10 (sequence identifier number 1-10), and the PCR primer comprises forward and reverse PCR amplification primers of the rest six hemorrhagic fever pathogens, a human DNA internal reference and a reaction internal reference, and PCR amplification primers of the nine hemorrhagic fever pathogens and the human RNA internal reference, and has a gene sequence show as SEQ ID NO. 10-36. The kit and the detection method have the advantages of high specificity, sensitivity, flux and reliability, low cost, and no false negative results.
Owner:NINGBO HEALTH GENE TECHNOLOGIES CO LTD
Kit for simultaneously detecting four pathogenic bacteria and non-diagnostic detection method thereof
ActiveCN105950759AHigh detection sensitivityStrong specificityMicrobiological testing/measurementMicroorganism based processesTime efficientYersinia pestis
The invention discloses a kit for simultaneously detecting four pathogenic bacteria Yersinia pestis, Frencisella tularensis, Burkholderia pseudomallei and Brucella by using fluorescent quantitative PCR (polymerase chain reaction) and a non-diagnostic detection method. The kit comprises specific primers and probes corresponding to the four pathogenic bacteria. The kit disclosed by the invention is convenient to use, and has the advantages of low reagent consumption, low cost, high detection specificity and high sensitivity. The detection method can detect four pathogenic bacteria for one sample, thereby greatly simplifying the operational process, reducing the repetitive operation steps, saving the time, reducing the labor consumed by repetitive operation, effectively saving the cost and implementing quick screening.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Kit for synchronously detecting twenty-two respiratory tract pathogens and detection method of kit
ActiveCN103074451BEnsuring Quality JudgmentsImprove detection efficiencyMicrobiological testing/measurementMicroorganism based processesPositive controlReverse transcriptase
The invention discloses a kit for synchronously detecting twenty-two respiratory tract pathogens and a detection method of the kit. The kit comprises DEPC (diethylpyrocarbonate) water, a 5*RT (reverse transcription) buffer, a reverse transcription primer, a reverse transcriptase, an X solution, a 10*PCR (polymerase chain reaction) buffer, a PCR primer, a 25mM magnesium chloride solution, a DNA (deoxyribonucleic acid) polymerase and a positive control, and is characterized in that the reverse transcription primer comprises RT amplification primers of the nine respiratory tract pathogens, and a reverse primer of a human RNA (ribonucleic acid) internal reference, and has a gene sequence shown as SEQ ID NO. 1-6 (sequence identifier number 1-6), and the PCR primer comprises forward and reverse PCR amplification primers of the rest thirteen respiratory tract pathogens, a forward and reverse amplification primer of a human DNA internal reference, a forward and reverse PCR amplification primer of a reaction internal reference, PCR amplification primers of the nine respiratory tract pathogens, and a PCR amplification primer of the human RNA internal reference, and has a gene sequence shown as SEQ ID NO. 7-44. The kit and the detection method have the advantages of high specificity, sensitivity, flux and reliability, low cost, and no false negative results.
Owner:NINGBO HEALTH GENE TECHNOLOGIES CO LTD
Heat laser strip device
PendingCN109148369AGuaranteed light transmissionSolve easy pollutionSemiconductor/solid-state device manufacturingLaser beam welding apparatusEngineeringLaser
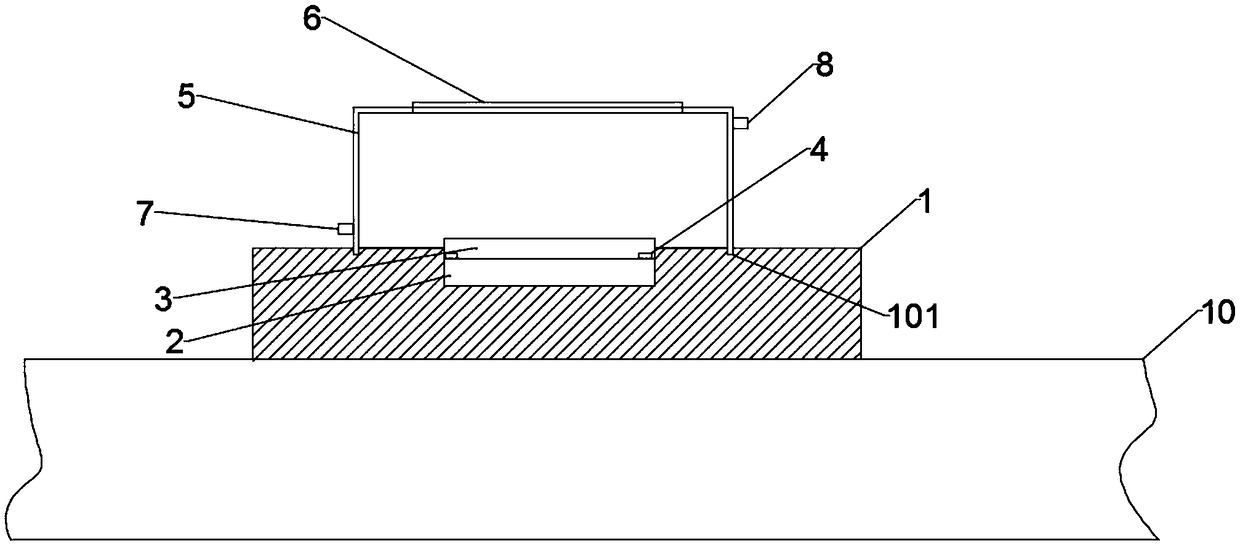
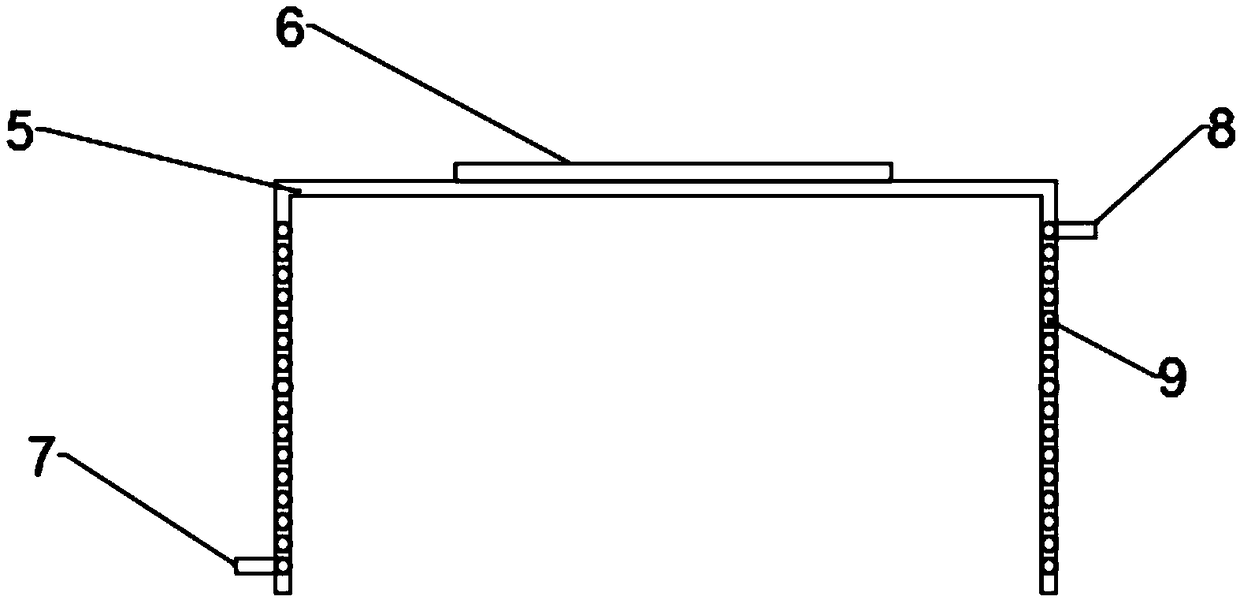
A laser stripping apparatus is disclose, include a laser strip apparatus body, A laser stripping apparatus body include a mobile platform arranged at a lower end, the mobile platform is provided witha heating base, A sample table is arranged on the heating base, the heating base comprises an electric heating device arranged below the sample table and used for heating the sample table, A temperature sensor for detect that temperature of the sample table is also arranged at the low end of the sample table, and a heat insulating cover is cover above the sample table, and a heat insulating transparent window convenient for the laser to pass through is arranged at the position corresponding to the top end of the heat insulating cover and the sample table. The heating laser stripping apparatusof the present invention provides a suitable temperature environment for stripping GaN epitaxial wafers, solves the problem of residual stress of GaN epitaxial wafers grown on a sapphire substrate, and avoids the occurrence of easy fragmentation during stripping.
Owner:SINO INNOV SEMICON (PKU) CO LTD
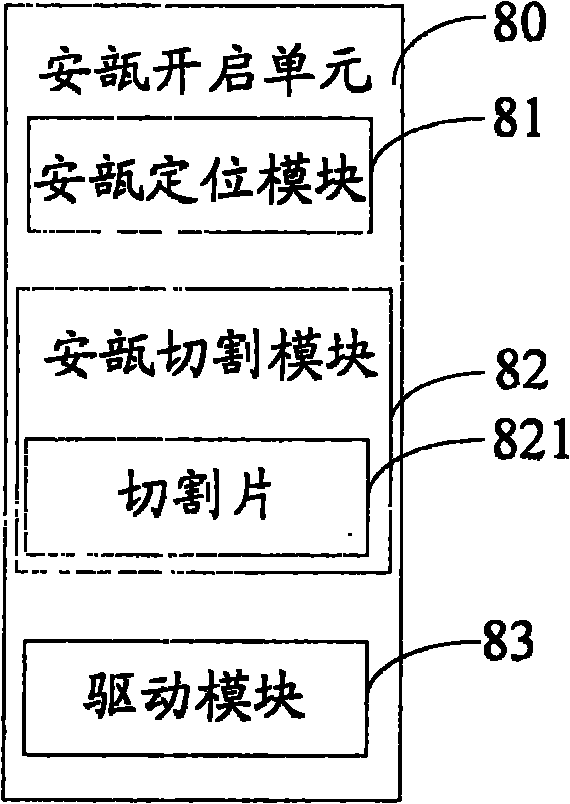
Body fluid analytical detection device for medical examination
PendingCN110840466ASolve the problem that two-way operation is required and blood is easily contaminatedSolve easy pollutionSurgeryVaccination/ovulation diagnosticsPhysicsCollection sample
The invention relates to the technical field of medical examination equipment, provides a body fluid analytical detection device for medical examination and aims to solve problems of simplicity in operation, few detection types, low detection efficiency, detection cost increase and proneness to contamination of body fluid collection samples due to separated puncture and drawing in blood sampling in application of an existing body fluid analytical detection device. The body fluid analytical detection device comprises a base, a frame and a main case in sequential arrangement from bottom to top.A puncturing and drawing mechanism is mounted on the front wall of the main case, and a liquid storage box, a drain tube, a detection liquid box, a dropper and a detection stage are sequentially arranged in an inner cavity of the main case from top to bottom. The drain tube is penetratingly connected with the liquid storage box and the detection liquid box, a liquid outlet valve II is mounted on the drain tube, the dropper is penetratingly arranged on the bottom wall of the detection liquid box, and a liquid outlet valve I is mounted on the dropper. The body fluid analytical detection device is especially applicable to analytical detection of body fluid of patients and has a high social use value and a promising application prospect.
Owner:刘艳
Automatic transfusion medicine dispensing equipment and automatic transfusion medicine dispensing method
ActiveCN101804225BSolve easy pollutionReduce riskInfusion devicesPharmaceutical product form changeBottlePollution
The invention provides automatic transfusion medicine dispensing equipment, which belongs to the technical field of transfusion medicine dispensation. The automatic transfusion medicine dispensing equipment can automatically extract liquid in an ampoule, medicine powder or water aqua in a powder bottle, and mother liquid in a mother liquid container to be mixed. The invention also provides an automatic transfusion medicine dispensing method. The equipment and the method in the invention can be used for realizing the effect of operating the liquid in the ampoule and the medicine powder or the water aqua in the powder bottle by a machine and mixing the liquid in the ampoule and the medicine powder or the water aqua in the powder bottle with the mother liquid, the problem of easy pollution of the medicine liquid is solved, the medicine dispensing risk is lowered, the disputes between doctors and patients can be reduced, and in addition, the invention saves labor and improves the nursing efficiency and quality.
Owner:山东卫邦智能机器人有限公司
Automatic feeding device
PendingCN104542325AStable deliveryAchieve compactionAnimal feeding devicesAvicultureEngineeringMaterial storage
The invention provides an automatic feeding device. The automatic feeding device comprises a material falling device arranged above a chicken raising cage in a chicken house, wherein the material falling device is connected with a material storage tank outside the chicken house by a charging device; the automatic feeding device further comprises an automatic walking device arranged in the chicken house; a mounting frame is arranged above the automatic walking device; the mounting frame is provided with an automatic discharging device and a power mechanism; the power mechanism is connected with the automatic walking device and the automatic discharging device; the material falling device is arranged above the mounting frame. The automatic feeding device can completely realize automatic charging and automatic feeding and the feeding is accurate; a feed feeding amount of each part in a material accepting groove is more uniform and the feed is not wasted, so that the maximum culture effect can be realized and the feed feeding amount of different phases can be intelligently controlled.
Owner:GUIZHOU JINGMING ANIMAL HUSBANDRY
Deep treatment method of high-salt organic sewage
ActiveCN108358363AImprove adsorption efficiencyGood removal effectWater/sewage treatment by irradiationWater/sewage treatment by magnetic/electric fieldsElectrolysisUltrafiltration
The invention discloses a deep treatment method of high-salt organic sewage. The method comprises the following steps: step one, carrying out pretreatment; step two, carrying out electroflocculation and precipitation; step three, carrying out ultraviolet-ozone-ultrasound combined reaction stage; step four, carrying out electrolysis treatment through three dimension electrodes; step five, filteringthrough three-dimensional graphene sponge; and step six, carrying out ultrafiltration-electrodialysis coupled desalinization. The method is free of biological treatment processes and high in processflexibility; compared with the common sewage treatment method, the treatment period is greatly shortened; meanwhile, the method is high in desalinization rate, simple to operate, high in efficiency, free of secondary pollution and low in cost, and is capable of completely removing organic matters, recycling the heavy metals and directly recycling the treated high-salt organic sewage.
Owner:江西楚杭环保科技有限公司
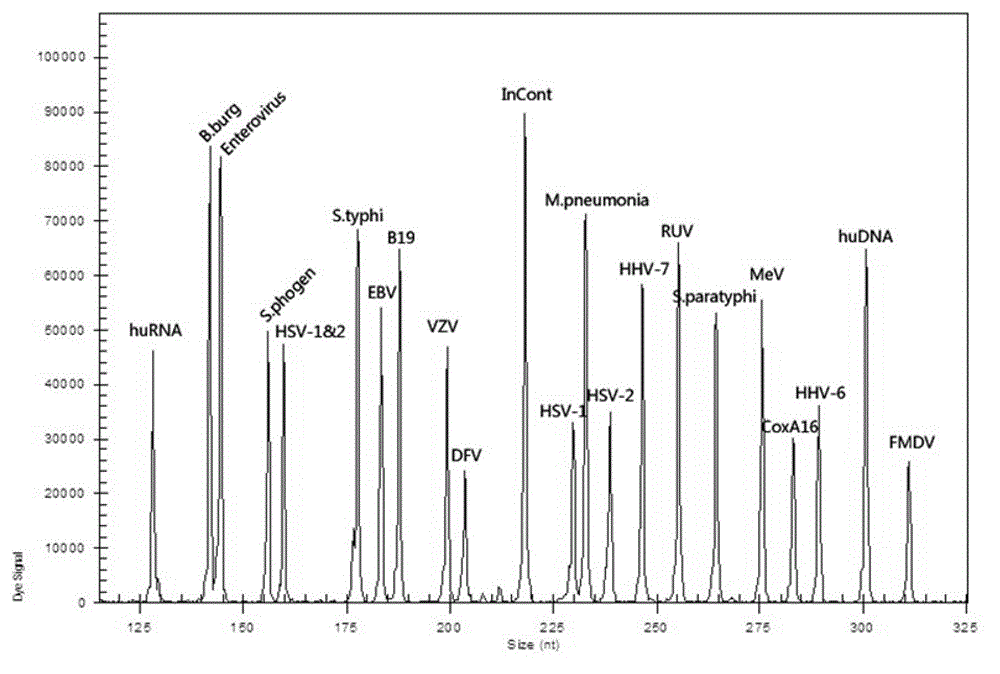
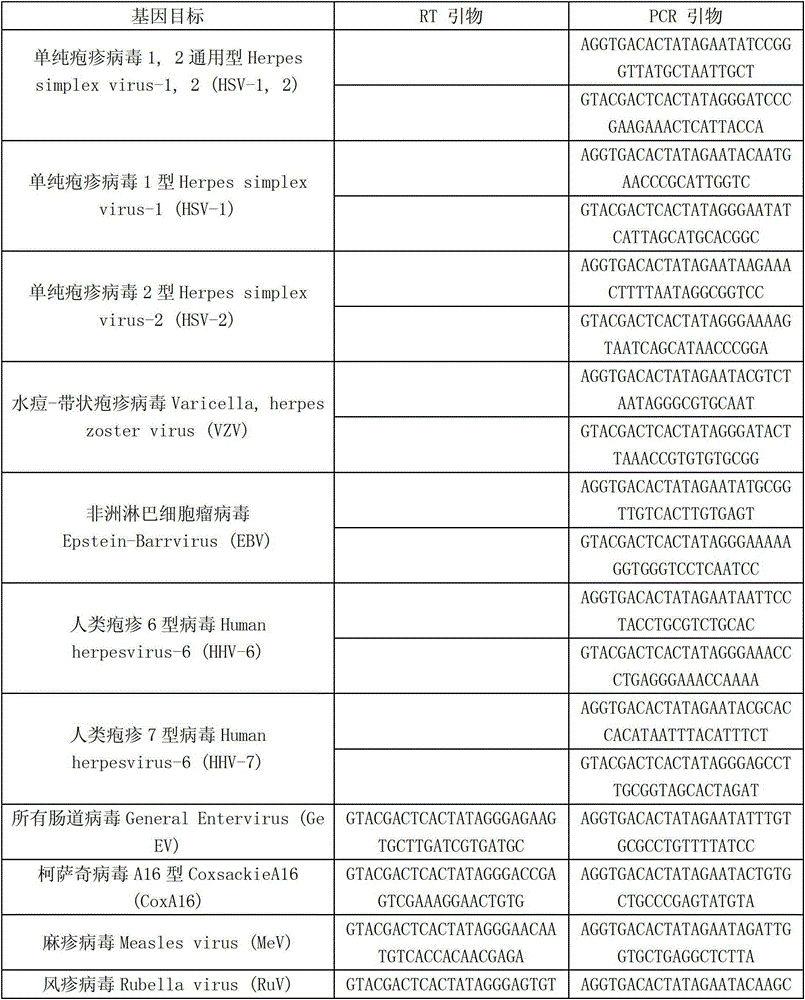
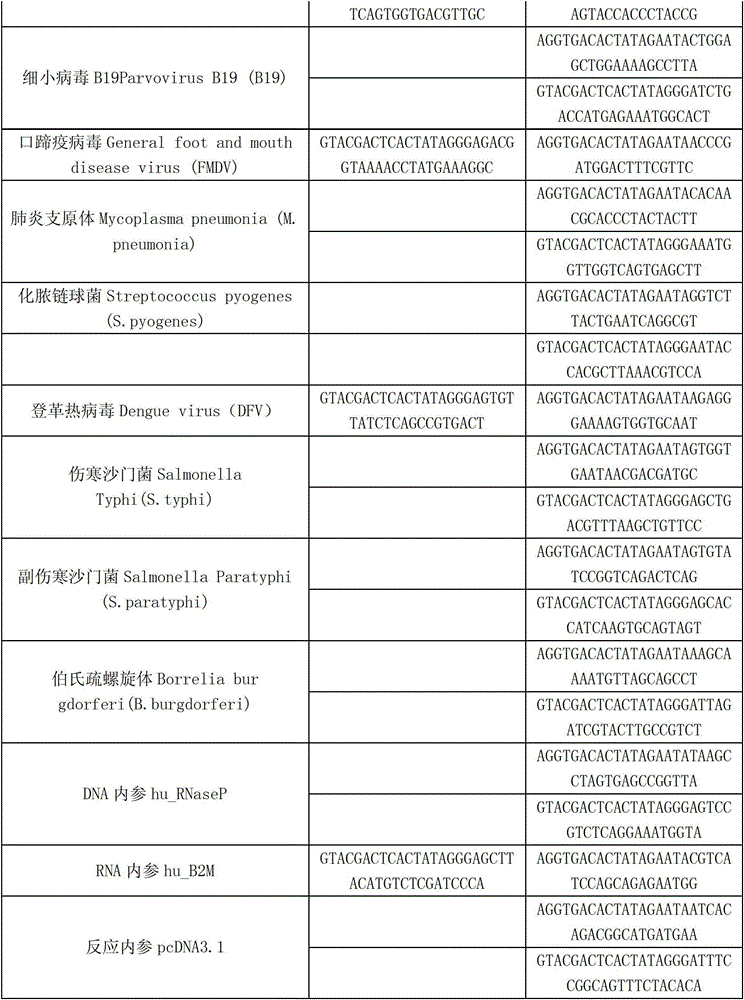
Kit capable of synchronously detecting eighteen kinds of fever with eruption pathogens and detection method thereof
ActiveCN103146841AEnsuring Quality JudgmentsImprove detection efficiencyMicrobiological testing/measurementMicroorganism based processesReverse transcriptasePolymerase L
The invention discloses a kit capable of synchronously detecting eighteen kinds of fever with eruption pathogens and a detection method thereof. The kit comprises diethylpyrocarbonate (DEPC) water, 5*reverse transcriptase (RT) buffer solution, reverse transcription primers, reverse transcriptase, X solution, 10* polymerase chain reaction (PCR) buffer solution, PCR primers, 25m M magnesium chloride solution, deoxyribonucleic acid (DNA) polymerase and positive reference substances. The kit is characterized in that the reverse transcription primers comprise five kinds of fever with eruption pathogens and ribonucleic acid (RNA) internal reference RT primers, and the sequences are shown as SEQ ID NO.1-NO.6. The PCR primers comprises the remaining thirteen kinds of fever with eruption pathogens, herpes simplex virus 1, 2 universal type, human DNA internal reference, forward and reverse PCR amplification primers of a reaction internal reference, and the five kinds of fever with eruption pathogens and PCR amplification primers of a human RNA internal reference. The gene sequence is shown as SEQ ID NO.7-NO.44. The kit has the advantages of being strong in specificity, high in sensitivity, high in flux, strong in reliability, low in cost, and free from false-negative results.
Owner:NINGBO HEALTH GENE TECHNOLOGIES CO LTD
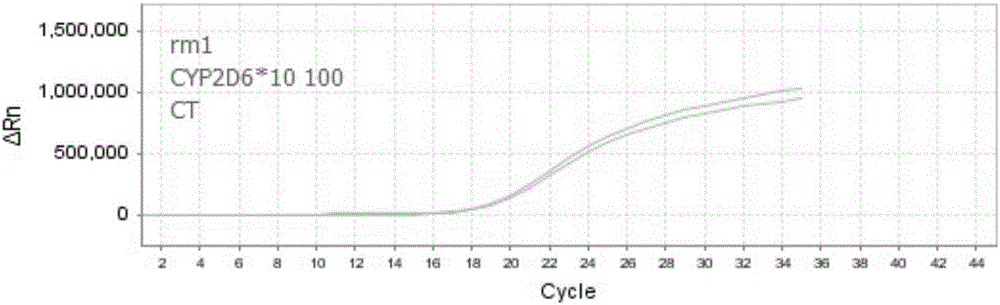
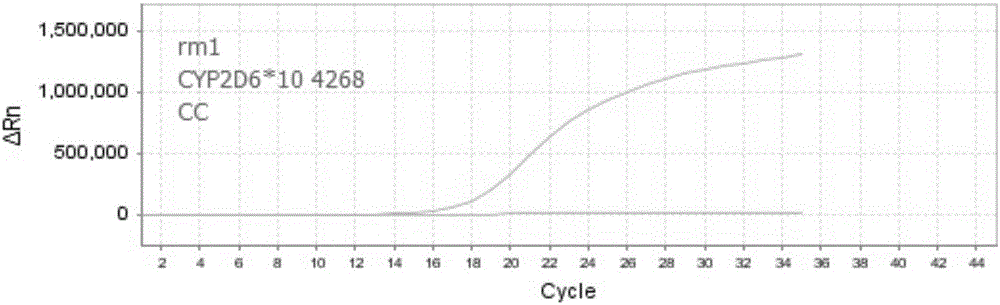
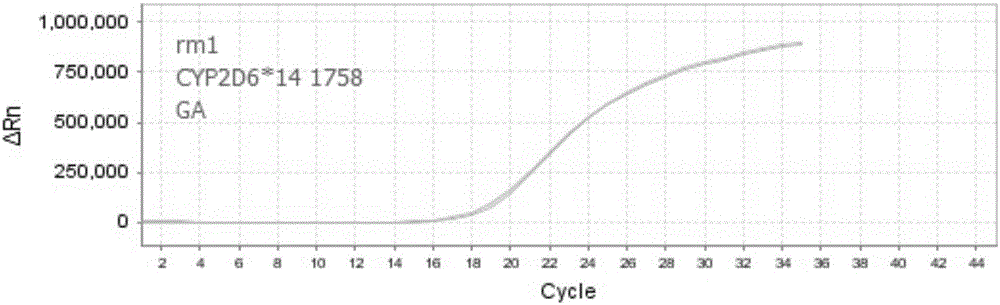
Nucleic acid, kit and method for detecting polymorphism of human CYP2D6 gene
ActiveCN106755360AHigh degree of automationEasy to useMicrobiological testing/measurementDNA/RNA fragmentationBiologyPollution
The invention discloses nucleic acid, a kit and a method for quickly detecting polymorphism of a human CYP2D6 gene, which are particularly used for detecting site polymorphism of CYP2D6*10 genes C100T and G4268C as well as a CYP2D6*14 gene G1758A. The detection kit for quickly detecting the polymorphism of the CYP2D6 gene by using real-time fluorescent quantitative PCR, has remarkable advantages of high specificity, high sensitivity, short experiment cycle, simplicity in operation, safety, no toxicity, low cost and the like; the detection method is simple and convenient to operate and high in automation degree; the operation process is greatly simplified, pollution in the operation process is reduced, and the detection result is accurate and reliable.
Owner:武汉海吉力生物科技有限公司
Nucleic acid, kit and method for detecting polymorphism of A118G locus of OPRM1 gene of human beings
ActiveCN106755320ASolve easy pollutionAvoid false positivesMicrobiological testing/measurementDNA/RNA fragmentationFalse positive rateNon specific
The invention discloses nucleic acid and a kit used for detecting polymorphism of A118G locus of OPRM1 gene of human beings, and establishes a method for detecting polymorphism of A118G locus of OPRM1 gene of human beings with strong specificity, high sensitivity, high accuracy and simple operation, and provides a guide scheme for individual dose administration of opioid, such as morphine, fentanyl, tramadol, oxycodone & aceta minophen and dolantin. The detection method provided in the invention adopts an operation in a completely-closed tube, the operation is simple, convenient and rapid, the detection result can be directly obtained by detecting the fluorescent signal value in the PCR process, and no PCR post-treatment or electrophoresis detection is needed, thereby overcoming the difficult problems of easy pollution of the traditional PCR technology, easy false positive rate, and capability of effectively avoiding non-specific amplification, and being applicable to detection of large-scale samples.
Owner:武汉海吉力生物科技有限公司
Method for preparing prostaglandin E1 by using gene engineering cyclooxygenase-1 and gene engineering prostaglandin E synthetase-1
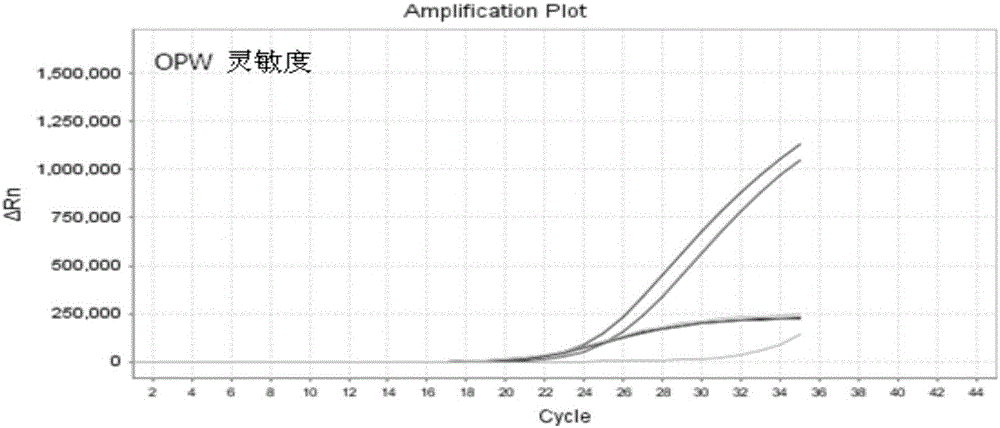
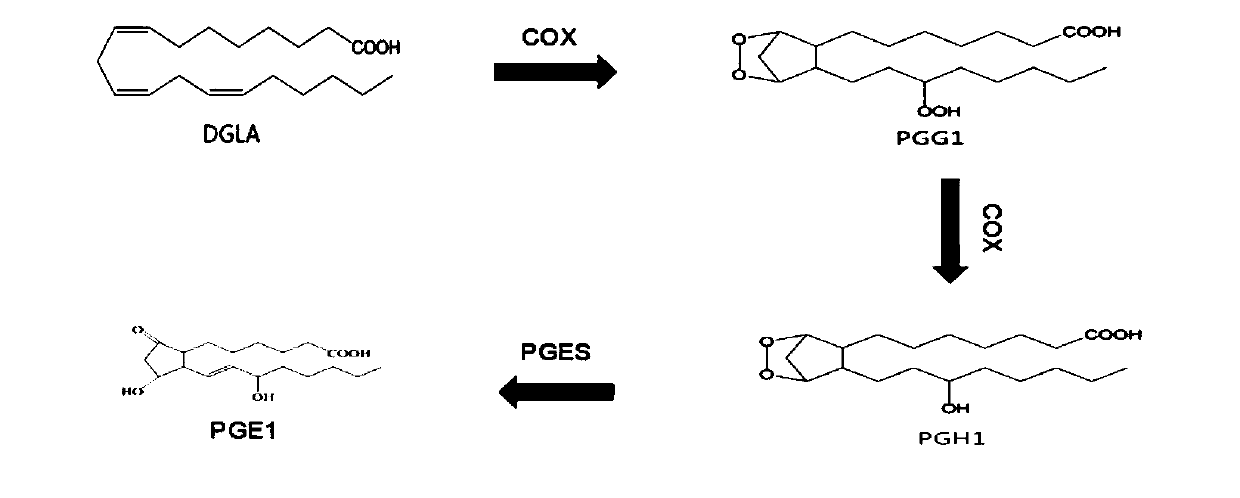
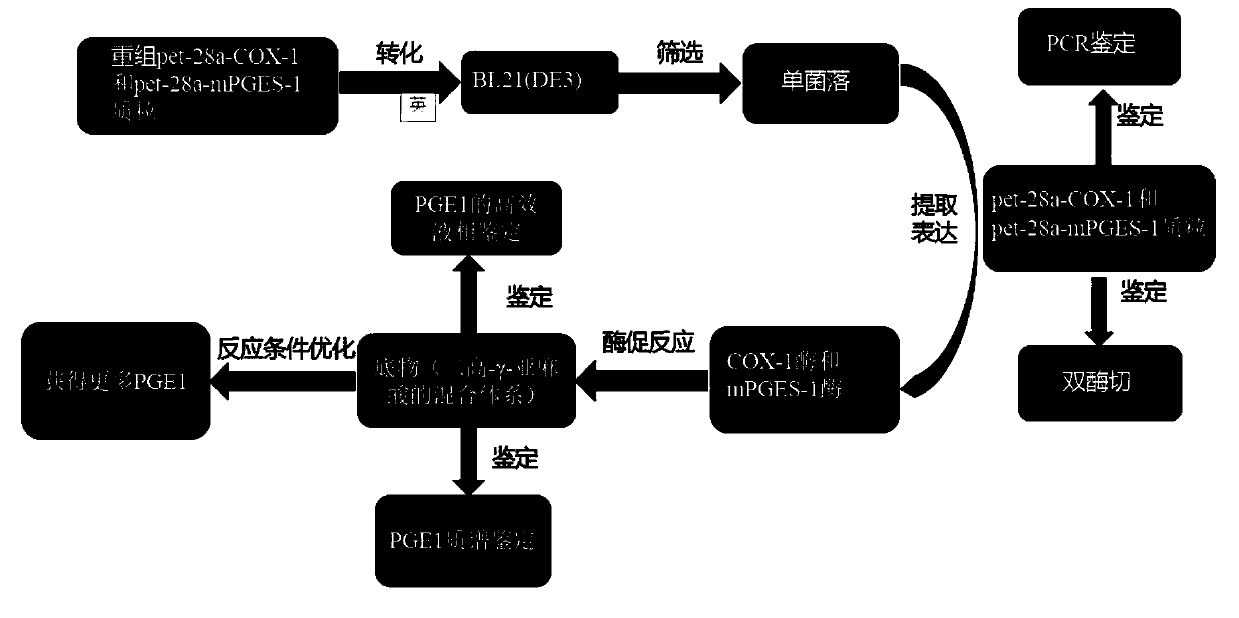
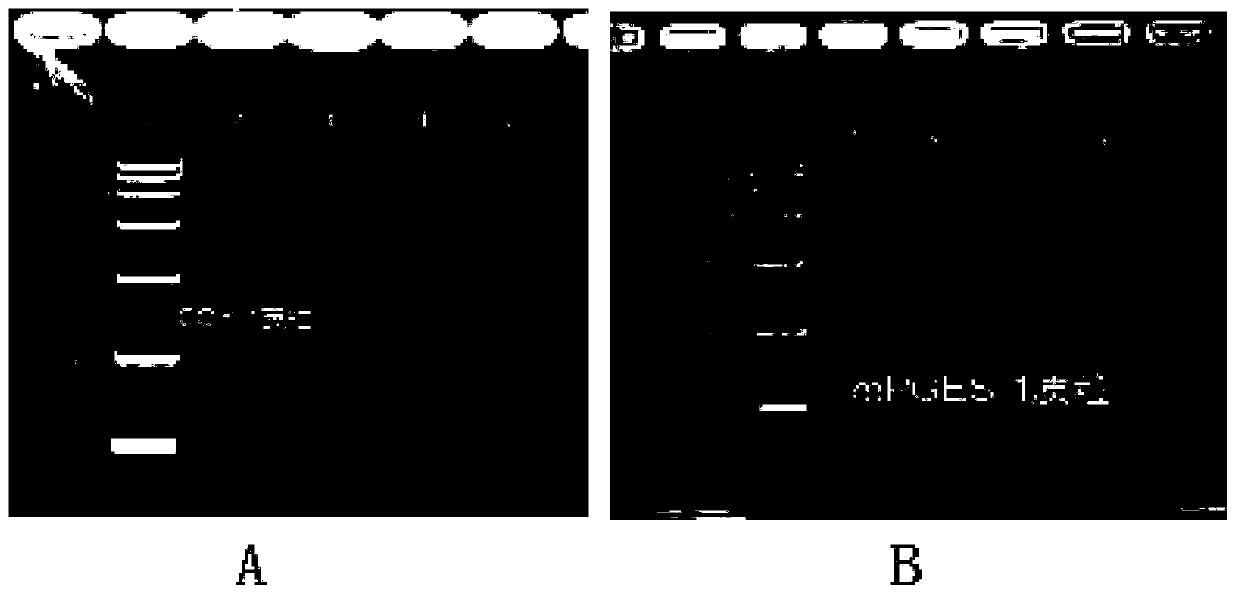
ActiveCN110951814AEasy to purifyEfficient use ofOxidoreductasesIsomerasesEscherichia coliProkaryotic expression
The invention discloses a method for preparing prostaglandin E1 by using gene engineering cyclooxygenase-1 and gene engineering prostaglandin E synthetase-1, and belongs to the field of bioengineering. The method comprises the following steps: expressing related enzymes required by prostaglandin E1 synthesis by a prokaryotic expression means, namely cyclooxygenase-1 (COX-1) and prostaglandin E synthetase-1 (mPGES-1); and reacting the enzymes with a substrate to synthesize the prostaglandin E1. By the method, a large amount of prostaglandin synthetase can be expressed, the prostaglandin E1 is synthesized, and the problem that tissue enzymes taken from living bodies are easy to pollute in industrial production can be solved. Meanwhile, the enzyme type expressed by a prokaryotic expression issingle, the concentration of the expressed enzyme is high, organic impurities in an escherichia coli system are few, and the impurities of a product after enzymatic reaction are few, so that the purification and utilization of the prostaglandin E1 are facilitated, and a new path is opened up for the artificial synthesis of the prostaglandin E1.
Owner:CHANGCHUN UNIV OF SCI & TECH
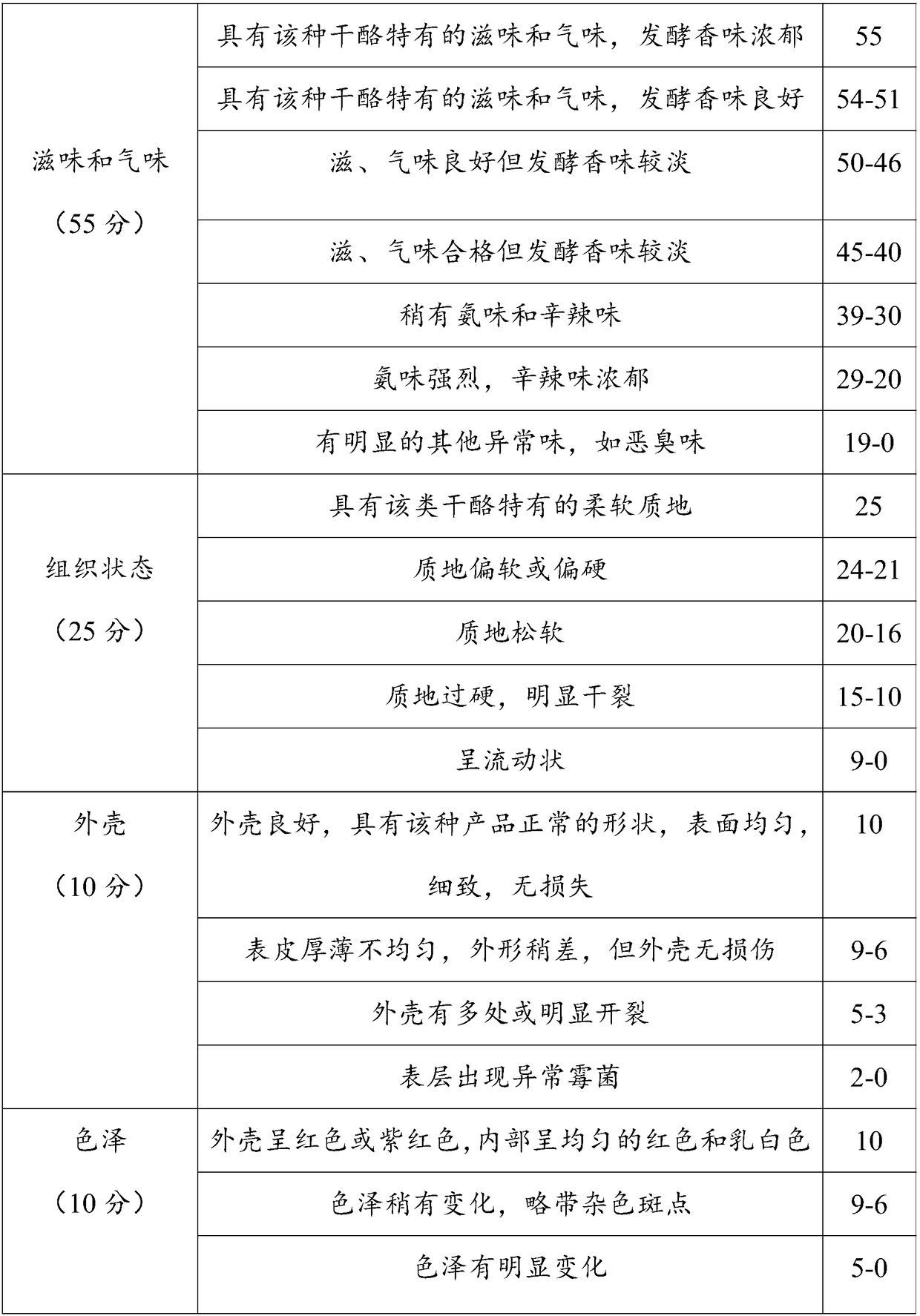
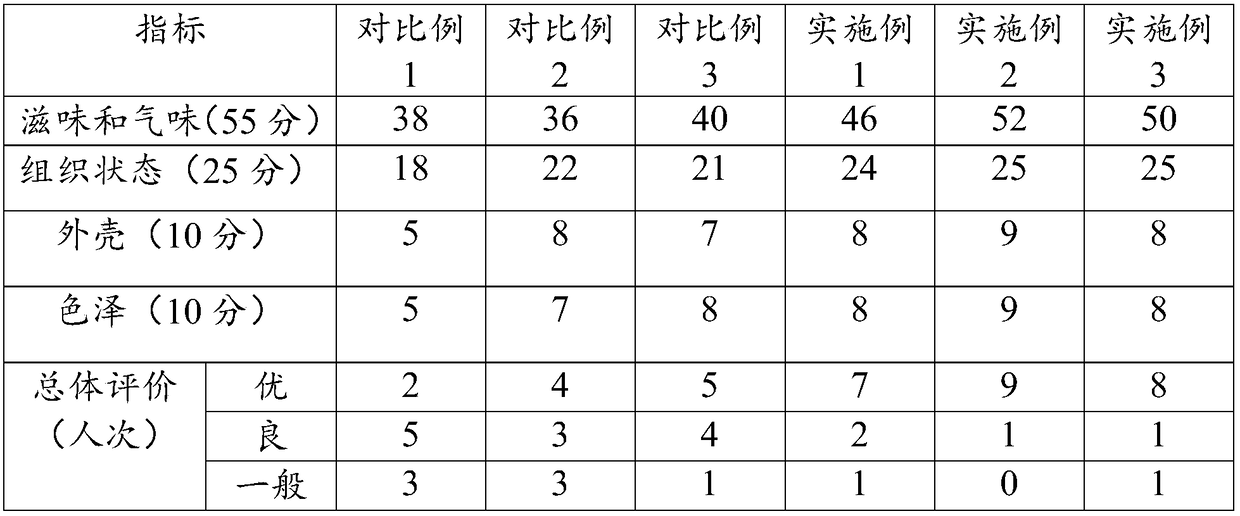
Monascus cheese and preparation method thereof
InactiveCN109430417AResolve flavorAddresses defects such as deterioration of the organizationCheese manufactureLactic acid bacteriumFlavor
The invention discloses monascus cheese and a preparation method thereof. The preparation method comprises the following steps of (1) performing direct vat set inoculation: inoculating sterilized rawmilk with a lactic acid bacterium fermenting agent and a monascus maturing agent, and performing pre-acidification until the pH value is 6.3-6.4; (2) adding chymosin: adding the chymosin to obtained materials, performing stirring for 1-3min, and then controlling the standing time to be 7-15min so as to obtain curd; (3) performing cutting treatment: performing cutting treatment on the curd, performing light stirring for 1min, performing standing for 10min, and performing repeating for 3 times so as to obtain curding blocks; (4) flushing the curding blocks: discharging out whey from the curdingblocks, and flushing the curding blocks with sterile water until the pH value of the curding blocks is 5.4-5.7; and (5) performing demolding: putting the curding blocks into molds, performing shaping,performing turning over at regular intervals, performing demolding, performing soaking in a saline solution, performing airing and performing maturing. According to the monascus cheese prepared by the preparation method disclosed by the invention, mild flavor is given to the cheese, the monascus cheese is fine and smooth in mouth feel, the preparation technology is simple, and the monascus cheeseis convenient in industrial production and popularization.
Owner:BRIGHT DAIRY & FOOD
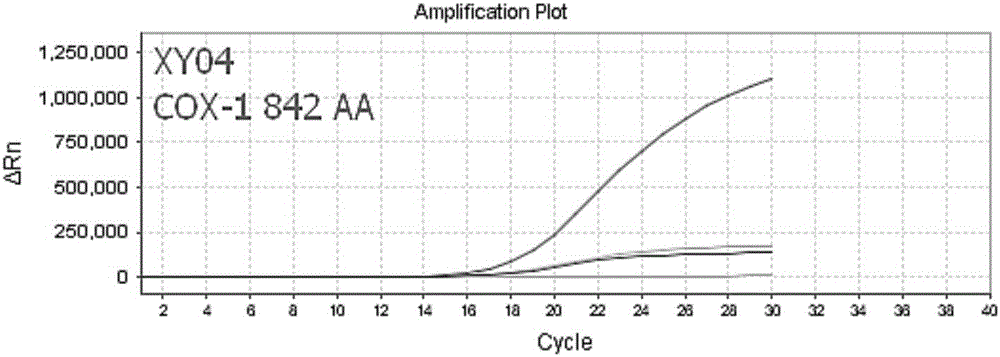
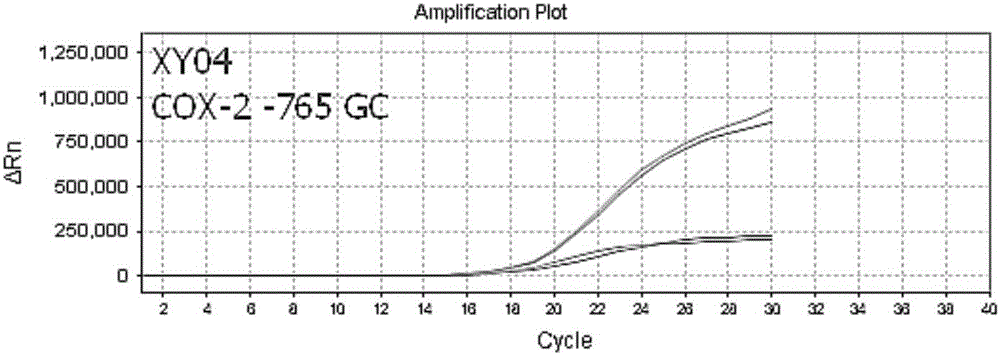
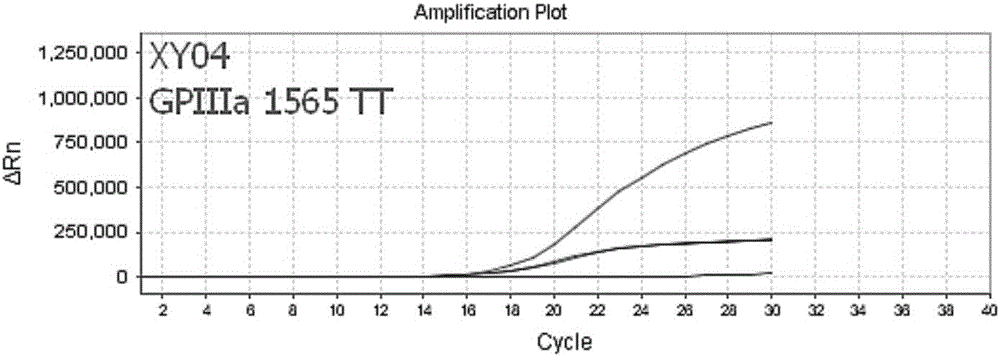
Nucleic acid, kit and method used for detecting polymorphism of COX-1, COX-2 and GPIIIa genes
ActiveCN106834434ASolve easy pollutionAvoid false positivesMicrobiological testing/measurementDNA/RNA fragmentationAspirinElectrophoresis
The invention discloses nucleic acid and a kit used for detecting polymorphism of COX-1, COX-2 and GPIIIa genes as well as detecting method for detecting polymorphism of the COX-1, COX-2 and GPIIIa genes, and the method is strong in specificity, is high in sensitivity, is high in accuracy and is simple to operate. The detected results can provide guidance meaning for aspirin resistance. The detecting method provided by the invention adopts completely closed-tube operation, is simple to operate, is convenient and quick, obtains the detected results by directly detecting a fluorescence signal value in a PCR process, PCR post-treatment or electrophoresis detection is not needed, the problems such as easy pollution and false positive results in the conventional PCR technology are overcome, the nonspecific amplification problem can be effectively avoided, and therefore, the nucleic acid, the kit and the method are suitable for large-scale sample detection.
Owner:武汉海吉力生物科技有限公司
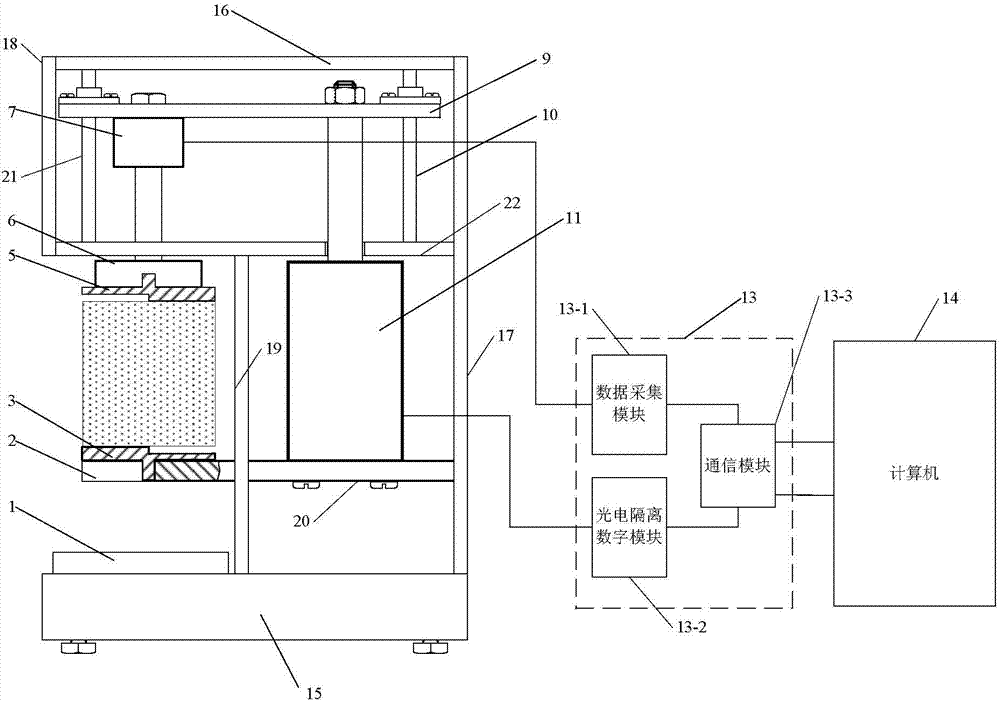
Vertical-type foundry sand five-intensity testing device and foundry sand intensity acquiring method based on device
InactiveCN103884595AOvercoming stiction and manual loading instabilityAvoid easy pollution problemsMaterial strength using tensile/compressive forcesData acquisition modulePressure sensor
The invention discloses a vertical-type foundry sand five-intensity testing device and a foundry sand intensity acquiring method based on the device, relates to the field of foundry sand, and aims to solve the problems that when the foundry sand intensity is tested by a horizontal-type hydraulic foundry sand intensity tester, the manual loading process is instable, hydraulic oil is likely to leak to pollute a tester and a foundry sand sample, and the foundry sand intensity testing result is inaccurate. A foundry sand sample is placed between an upper pressure head and a lower pressure head; a computer controls a linear motor to drive a loading force transmitting plate to drive the upper pressure head to apply pressure downward to the foundry sand sample by a communication module and an optoelectronic isolation digital module; a tension and pressure sensor monitors the variation of loading force on an upper pressure head seat in real time, and sends loading force data to the computer by a data acquisition module and the communication module; the computer calculates according to the loading force data to obtain the foundry sand intensity. The vertical-type foundry sand five-intensity testing device is suitable for testing on five intensities of foundry sand.
Owner:HARBIN UNIV OF SCI & TECH
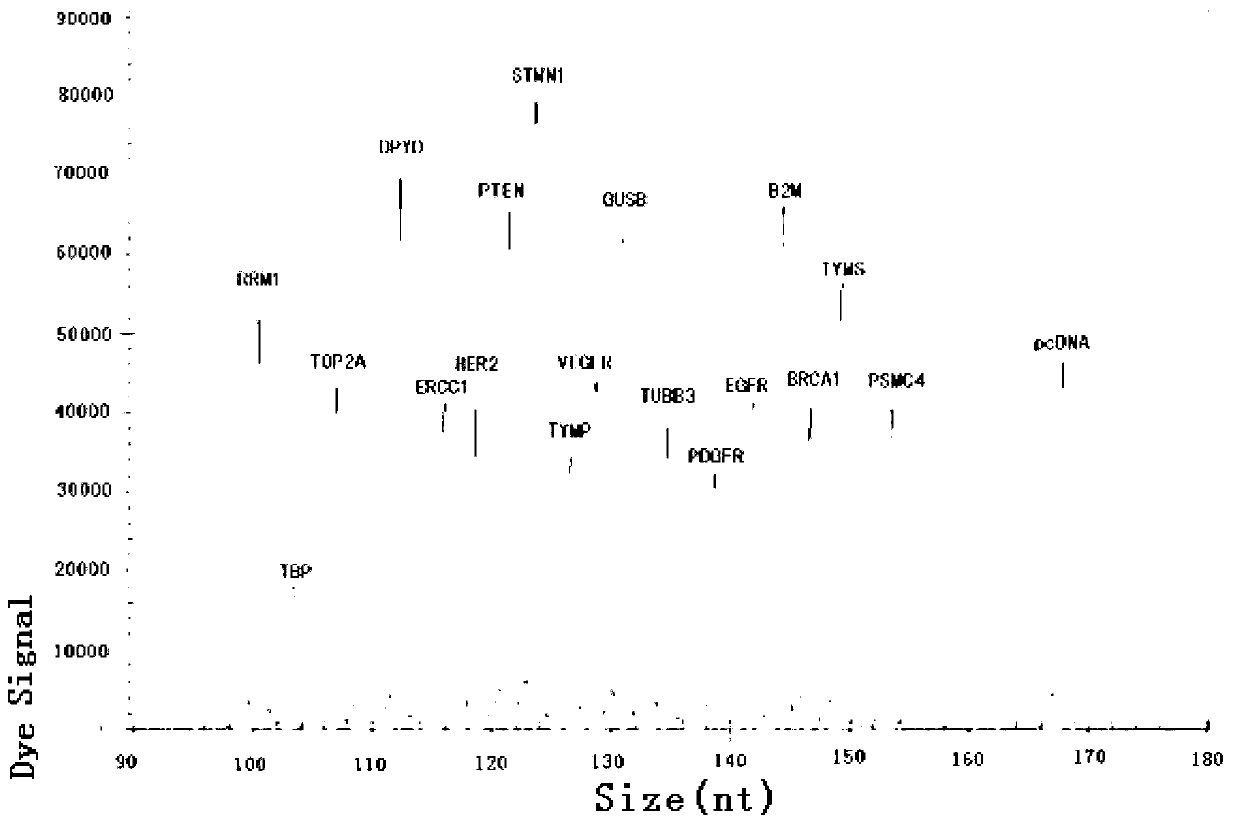
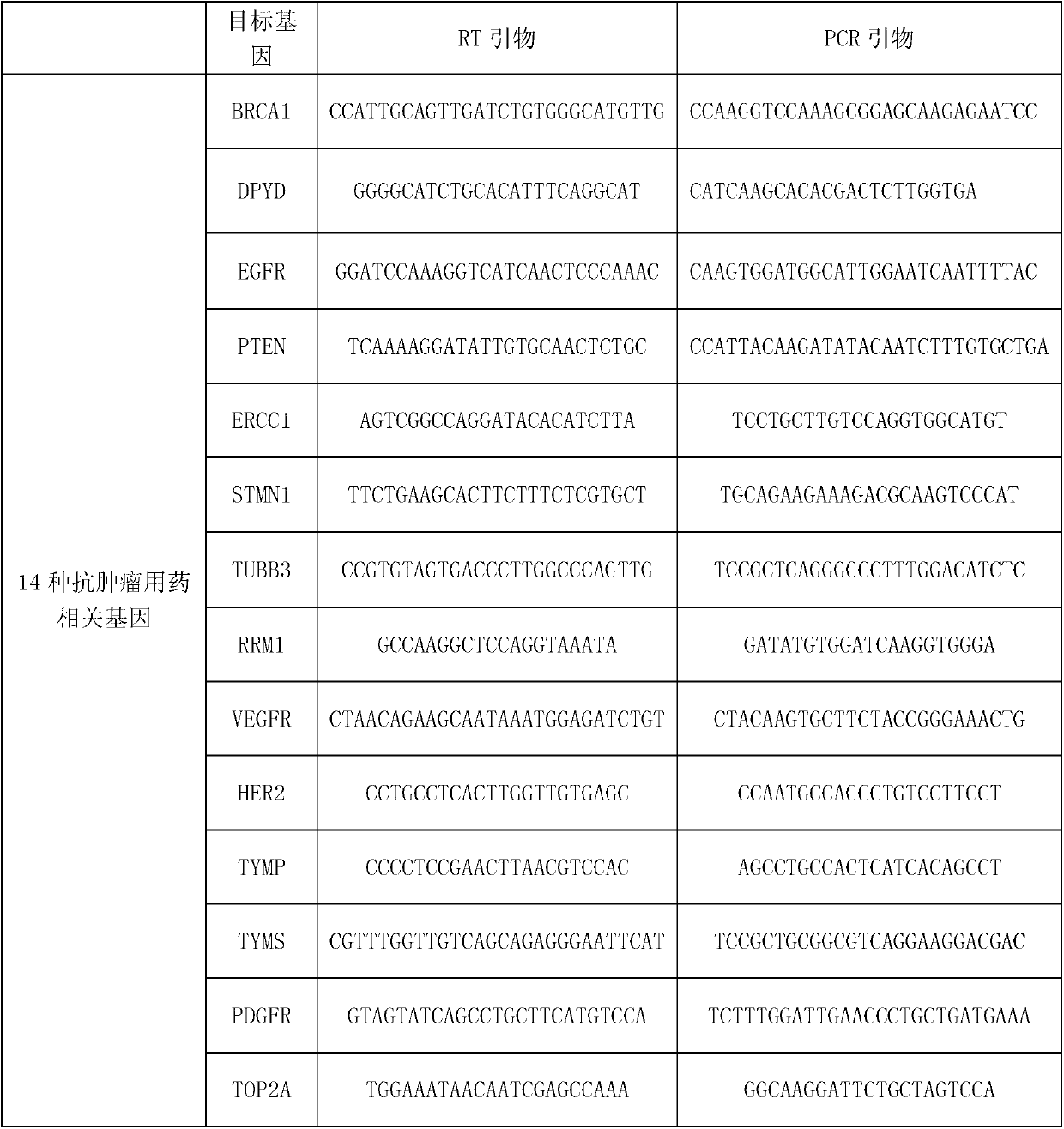
Kit for synchronously detecting related gene expression level of 14 antitumor drugs by using paraffin embedding biopsy sample, and detection method thereof
ActiveCN103305600AStrong specificityImprove detection efficiencyMicrobiological testing/measurementMaterial analysis by electric/magnetic meansPolymerase LGene expression level
The invention discloses a kit for synchronously detecting related gene expression level of 14 antitumor drugs by using a paraffin embedding biopsy sample, and a detection method thereof. The kit comprises DEPC (diethylpyrocarbonate) water, 5*RT buffer solution, a reverse transcription primer, a reverse transcription enzyme, an X solution, 10*PCR buffer solution, a PCR primer, 25mM magnesium chloride solution, DNA (deoxyribonucleic acid) polymerase and a positive reference substance; the reverse transcription primer comprises related genes of the 14 antitumor drugs, and RT amplification primers of an RNA (ribonucleic acid) internal reference; gene sequences are shown in SEQ ID NO.1 to NO.18; the PCR primer includes related genes of the 14 antitumor drugs, and positive and negative PCR amplification primers of a DNA internal reference; the gene sequences are shown in SEQ ID NO.19 to NO.38. The kit has the advantages of strong specificity, high sensitivity, high flux, strong reliability, low cost and absence of false-negative result.
Owner:NINGBO HEALTH GENE TECHNOLOGIES CO LTD
Nucleic acid, kit and method for rapid detection of C3435T polymorphism of ABCB1 gene
ActiveCN106755352AHigh degree of automationEasy to useMicrobiological testing/measurementDNA/RNA fragmentationC3435t polymorphismMicrobiology
The invention discloses a nucleic acid, kit and method for rapid detection of C3435T polymorphism of an ABCB1 gene. The nucleic acid comprises detection primers and a detection probe, wherein the detection primers comprise a wild downstream primer, a mutant downstream primer and a common upstream primer; a nucleotide sequence of the wild downstream primer is as shown in SEQ ID No.1, a nucleotide sequence of the mutant downstream primer is as shown in SEQ ID No.2, a nucleotide sequence of the common upstream primer is as shown in SEQ ID No.3, and a nucleotide sequence of the detection probe is as shown in SEQ ID No.4. The kit and method for real-time fluorescent quantitative PCR rapid detection of C3435T polymorphism of the ABCB1 gene have the advantages of being convenient to use, simple to operate and high in automatic degree, greatly simplifying the operation process and reducing pollution in the operation process, and the detection effect is good; the kit has the characteristics of high sensitivity, specificity, accuracy and precision.
Owner:武汉海吉力生物科技有限公司
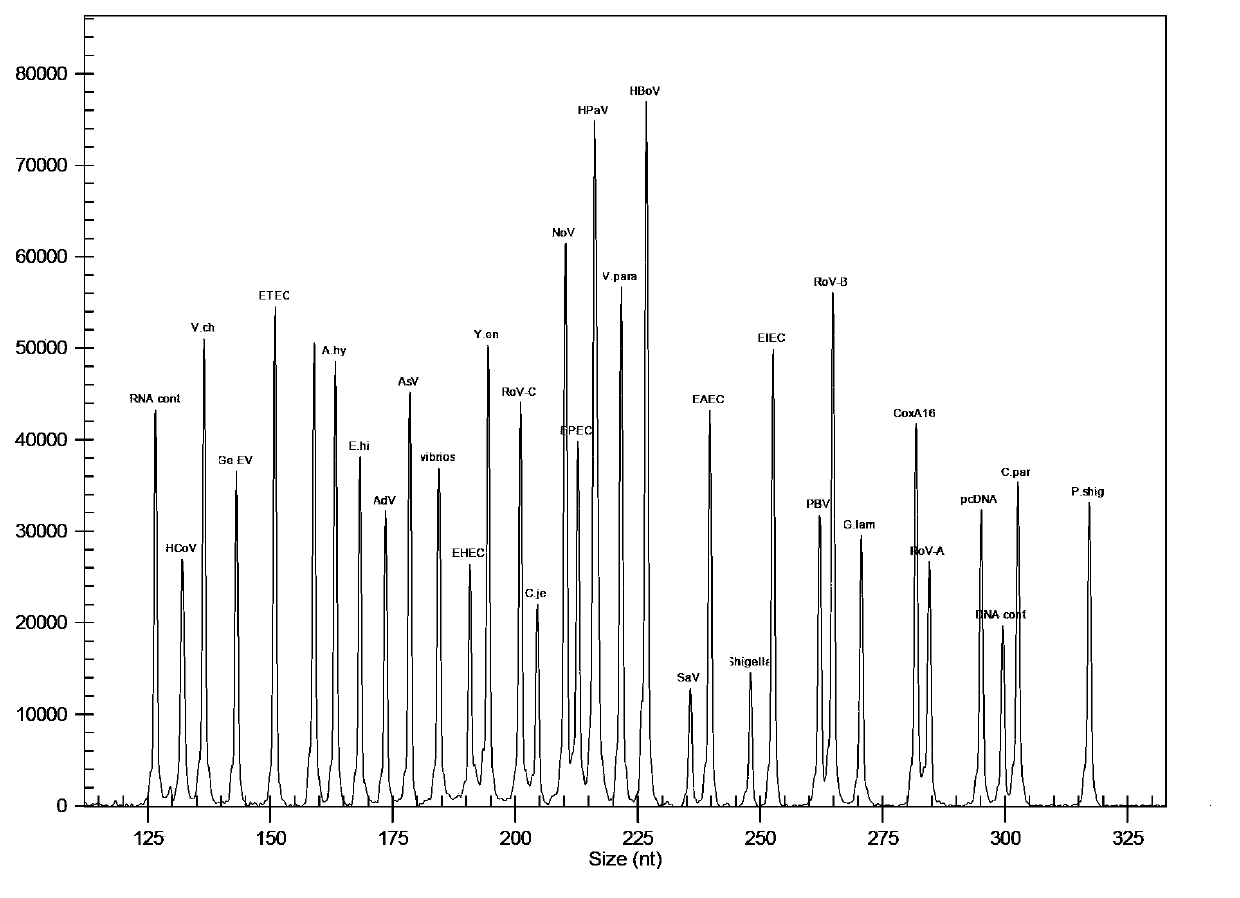
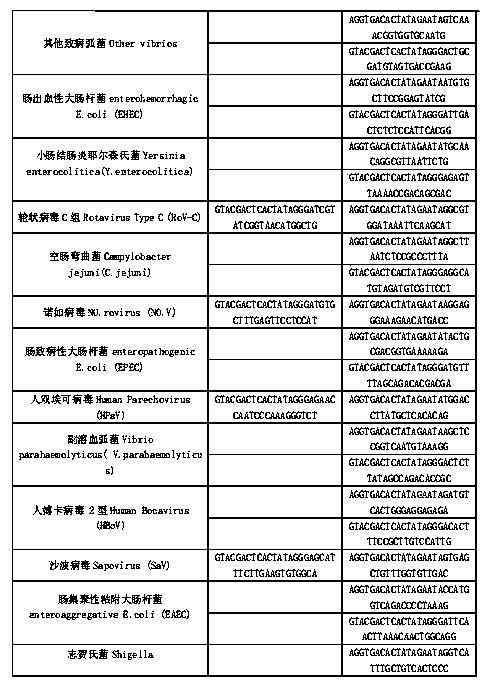
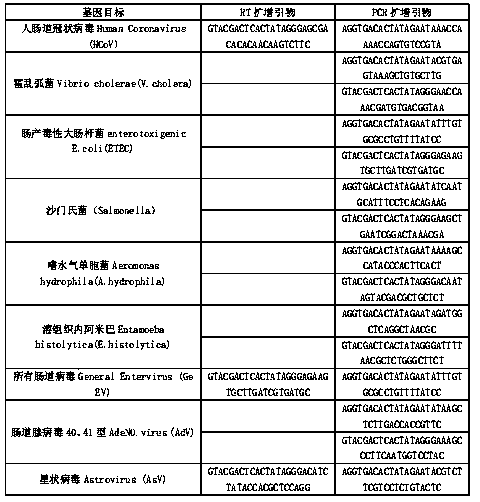
Kit for synchronously detecting thirty diarrhea pathogens and detection method of kit
ActiveCN103074450BStrong specificityImprove detection efficiencyMicrobiological testing/measurementMicroorganism based processesPositive controlReverse transcriptase
The invention discloses a kit for synchronously detecting thirty diarrhea pathogens and a detection method of the kit. The kit comprises DEPC (diethylpyrocarbonate) water, a 5*RT (reverse transcription) buffer, a reverse transcription primer, a reverse transcriptase, an X solution, a 10*PCR (polymerase chain reaction) buffer, a PCR primer, a 25mM magnesium chloride solution, a DNA (deoxyribonucleic acid) polymerase and a positive control, and is characterized in that the reverse transcription primer comprises RT amplification primers of eleven diarrhea RNA viruses and a human RNA (ribonucleic acid) internal reference, and has a gene sequence shown as SEQ ID NO. 1-12 (sequence identifier number 1-12), and the PCR primer comprises forward and reverse PCR amplification primers of the rest nineteen diarrhea pathogens, a human DNA internal reference and a reaction internal reference, and PCR amplification primers of eleven diarrhea RNA viruses and the human RNA internal reference, and has a gene sequence shown as SEQ ID NO. 13-66. The kit and the detection method have the advantages of high specificity, sensitivity, flux and reliability, low cost, and no false negative results.
Owner:NINGBO HEALTH GENE TECHNOLOGIES CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com