Plasmid carrying nanoparticle for preventing and treating influenza virus and preparation method thereof
A nanoparticle and plasmid technology, used in biochemical equipment and methods, gene therapy, antiviral agents, etc., can solve the problems of target gene mutation, target gene inactivation, deletion, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0146] The preparation of embodiment 1 nanoparticle
[0147] 1. Experimental method
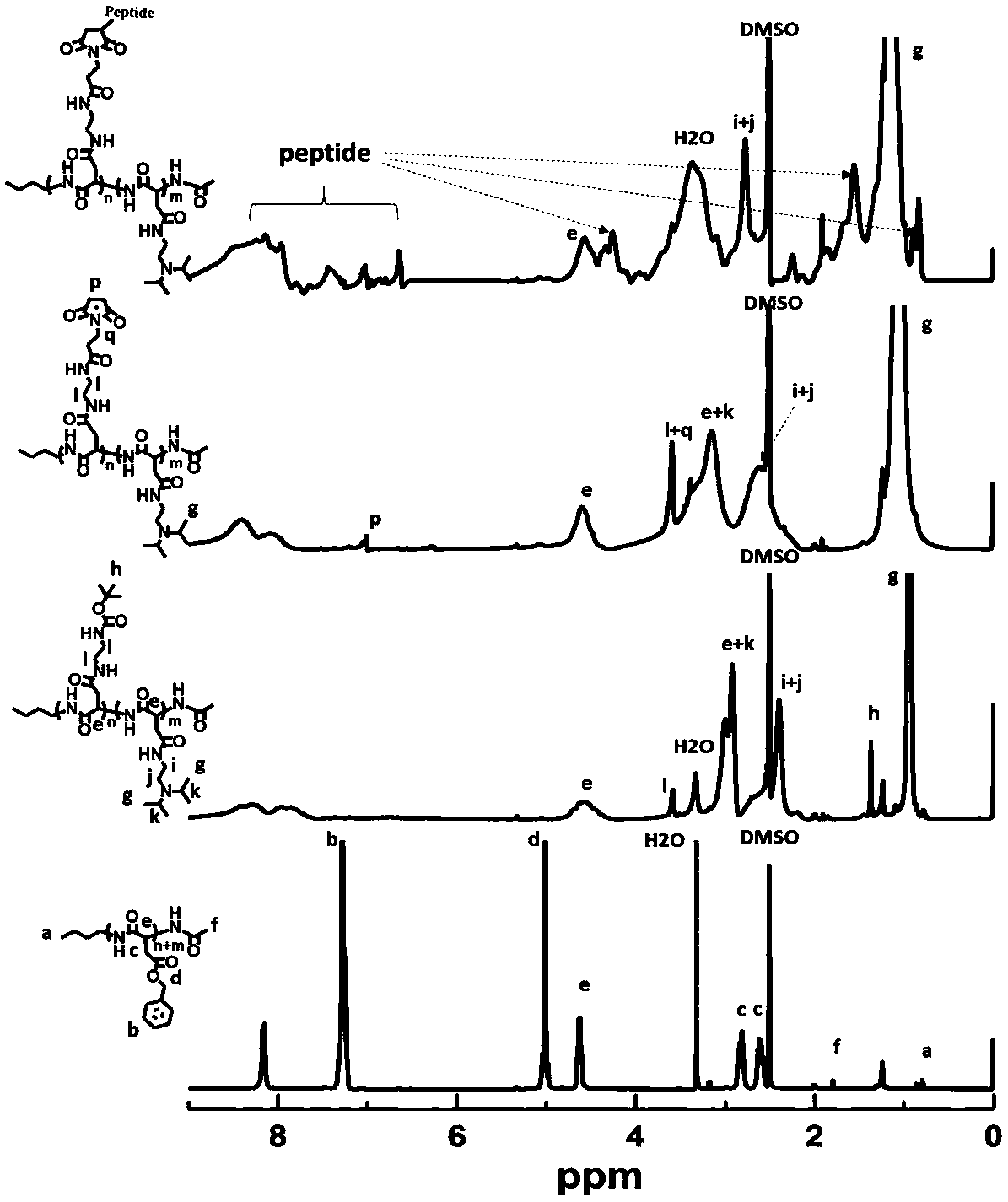
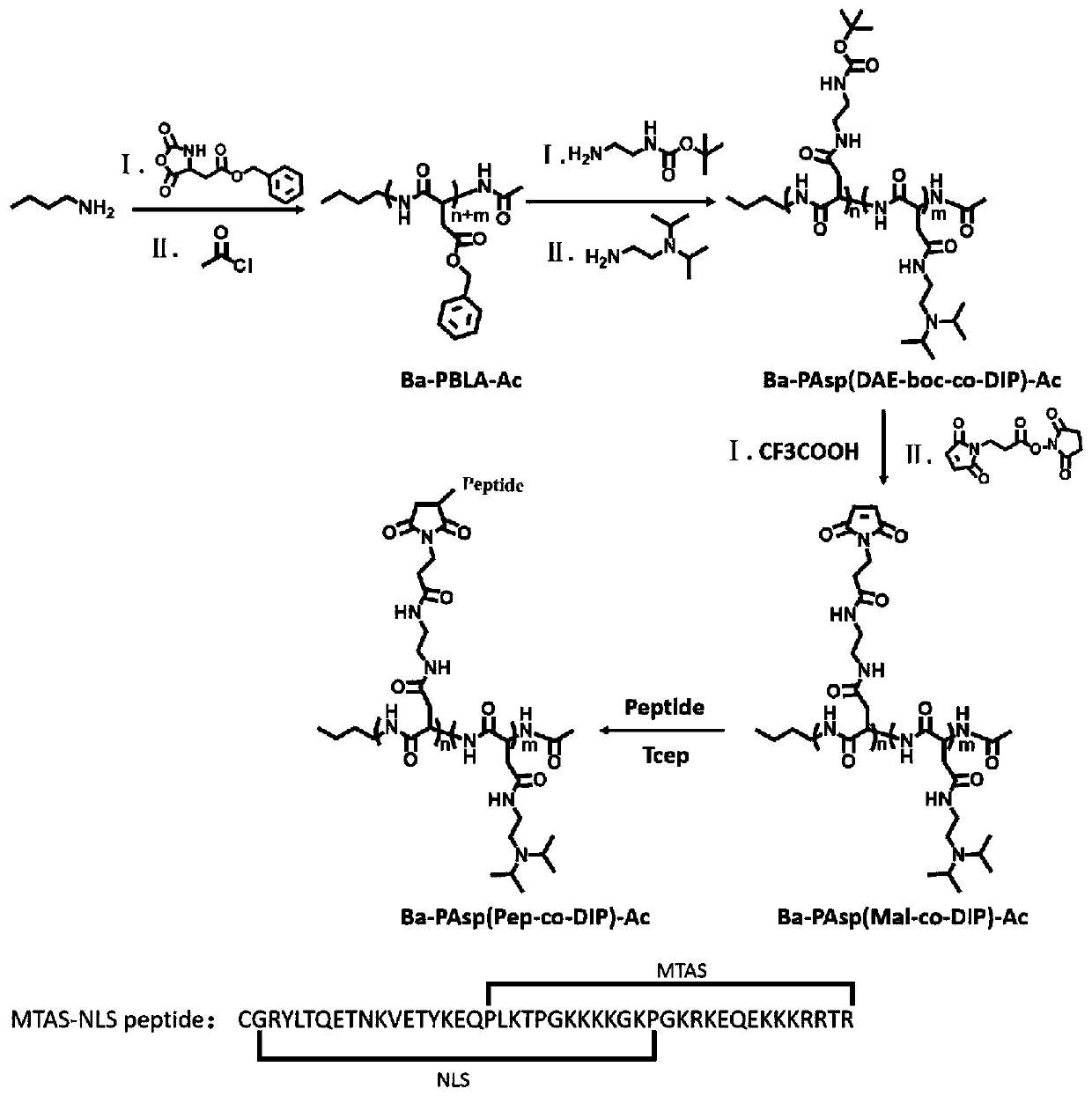
[0148] The synthetic route for the preparation of nanoparticles is shown in figure 1 shown.
[0149] 1. Synthesis of BLA-NCA monomer
[0150] (1) Synthesis of benzyl aspartate (BLA)
[0151] Prepare a single-necked bottle (500mL), add 100mL of anhydrous ether, then slowly add 10mL of H 2 SO 4 (98%), stirring vigorously while adding dropwise, after cooling to room temperature, add 100mL benzyl alcohol, after fully stirring, use the rotary evaporator to rotary evaporate ether. Add 13.3 g of aspartic acid (L-aspartic acid) in three times then to the reaction flask. Stir uniformly at room temperature for 24 hours, then add 200 mL of 95% ethanol, and add 50 mL of pyridine dropwise with a dropping funnel, stirring vigorously while dropping. Then freeze overnight in the refrigerator, pump the filtered solid, stir at 80°C, dissolve with deionized water, heat filter, and refrigerate the filtrat...
Embodiment 2
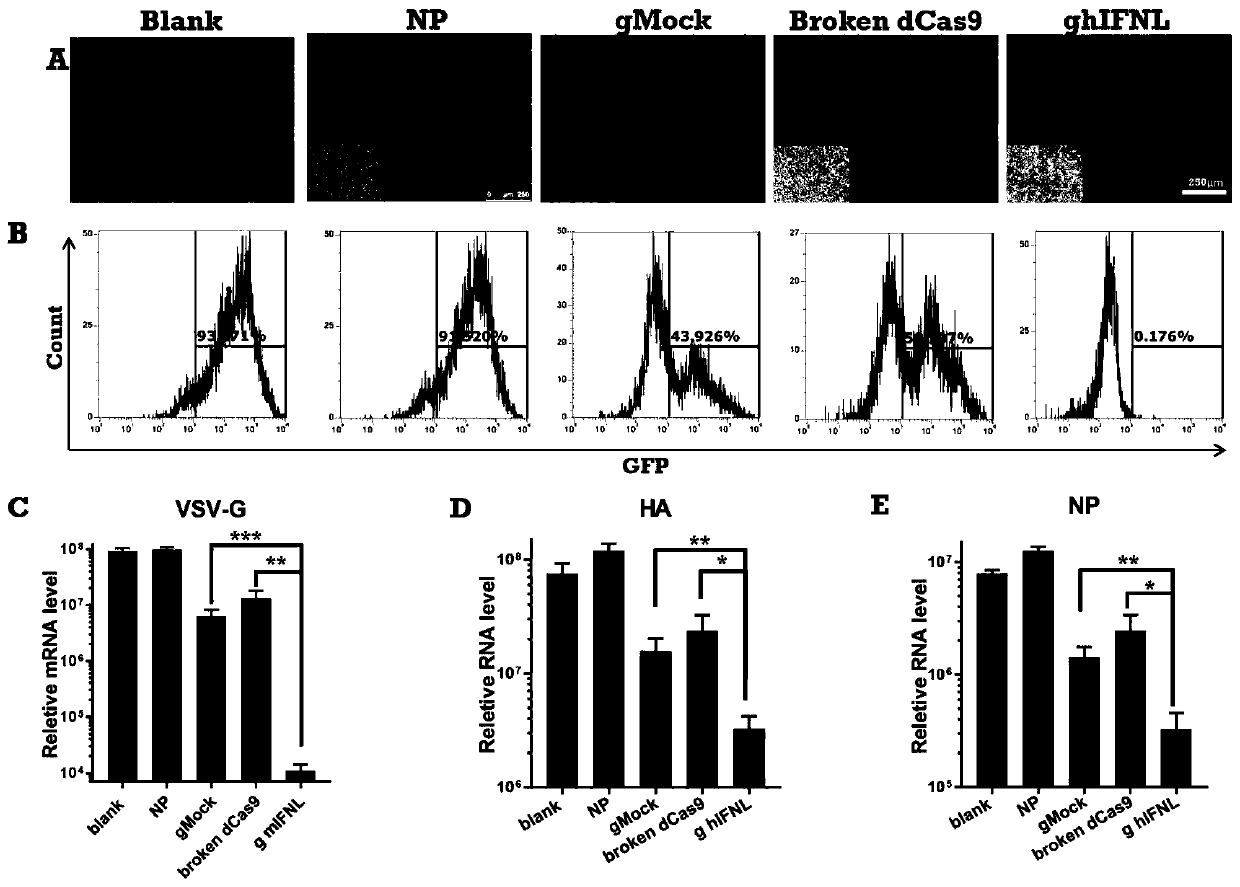
[0167] Example 2 Cellular uptake of nanoparticles
[0168] 1. Experimental method
[0169] 1. Mark the SP-dCas9-VPR plasmid
[0170] The plasmid SP-dCas9-VPR plasmid expressing dCas9 protein was incubated with YOYO-1 dye (Thermofisher) for 5 minutes, and the incubation ratio was carried out according to the official instructions, and then the SP-dCas9-VPR plasmid labeled with green fluorescent marker was obtained for subsequent cell uptake Experimental observation.
[0171] 2. Transfection of nanoparticles loaded with SP-dCas9-VPR plasmid
[0172] The SP-dCas9-VPR plasmid labeled with YOYO-1 and the Ba-PAsp(Pep-co-DIP)-Ac nanoparticles prepared in Example 1 were used to prepare the plasmid transfer carrier. The specific preparation process is as follows:
[0173] The polymer Ba-PAsp(Pep-co-DIP)-Ac was dissolved in a buffer solution of acetic acid and sodium acetate (PH=5.5, 0.1M) to 5 mg / mL, and the polymer solution and the plasmid solution were mixed at a mass ratio of 40 / ...
Embodiment 3
[0179] Example 3 Construction of up-regulated gRNA plasmid expressing IFNL gene
[0180] 1. Experimental method
[0181] 1. Design of gRNA
[0182] Query the target IFNL gene on the NCBI official website to obtain the full sequence. The first base of the obtained full sequence is the TSS site, the upstream query is -600bp, the downstream query is +100bp, and the obtained 700bp sequence is the gRNA target sequence. Design gRNA sequences, and design at least 5 gRNAs for each gene.
[0183] The target gene is the IFNⅢ gene, 4 members IFNL1 (IL-29), IFNL2 (IL-28a), IFNL3 (IL-28b), IFNL4 in humans, and 2 members IFNL2 (IL-28a) in mice , IFNL3 (IL-28b).
[0184] The gRNA is composed of an RNA sequence fragment that specifically recognizes the target region and a fixed backbone RNA sequence. The backbone sequence is: 5'-GUUUUAGAGCUAGAAAUAGCAAGUUAAAAUAAGGCUAGUCCGUUAUUCAACUUGAAAAAGUGGCACCGAGUCGGUG-3'(SEQ ID NO:5). The gRNA sequences described later are all related to the target Re...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com