Nucleic acid analysis method based on constant-temperature cross-catalyzed nuclease reaction
A nucleic acid analysis and nuclease technology, applied in the field of nucleic acid analysis based on constant temperature cross-catalyzed nuclease reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
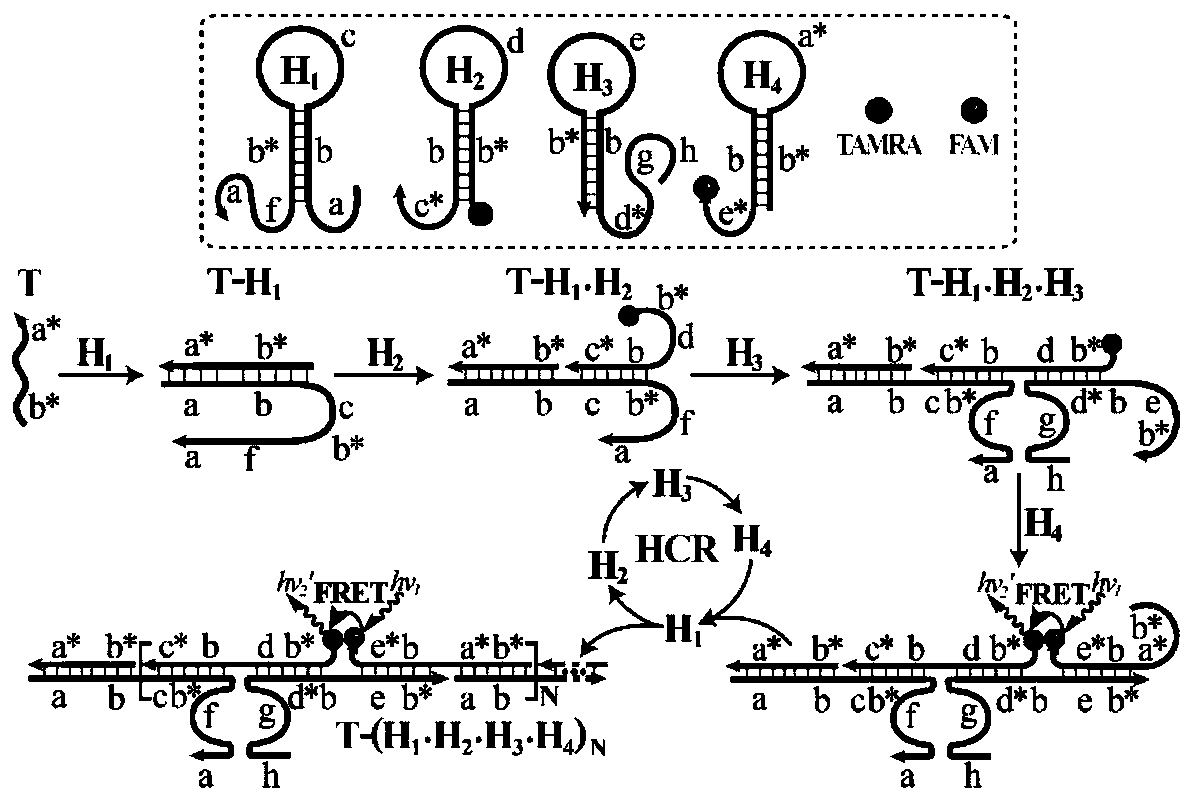
[0034] Design of DNA probes: Use NUPACK software to design relevant DNA probes, and entrust Sangon Bioengineering (Shanghai) Co., Ltd. to synthesize relevant nucleic acid sequences. When there is no target, ensure that the stem end of each hairpin has enough complementary base pairing to maintain its own stability and keep the fluorescence unchanged. However, when the target exists, it can trigger a constant temperature cross-catalyzed nuclease reaction and generate fluorescence. Resonance energy transfer to realize the detection of target objects. All DNA probe dry powders were first dissolved in phosphate buffer, and their absorbance was measured with a UV spectrophotometer to calculate the exact concentration. 2 ) Prepare all DNA probes to 4 μM, in PCR at 95° C. for 5 minutes, and at 25° C. for 2 hours to form stable hairpins. All reactions were carried out in HEPES buffer.
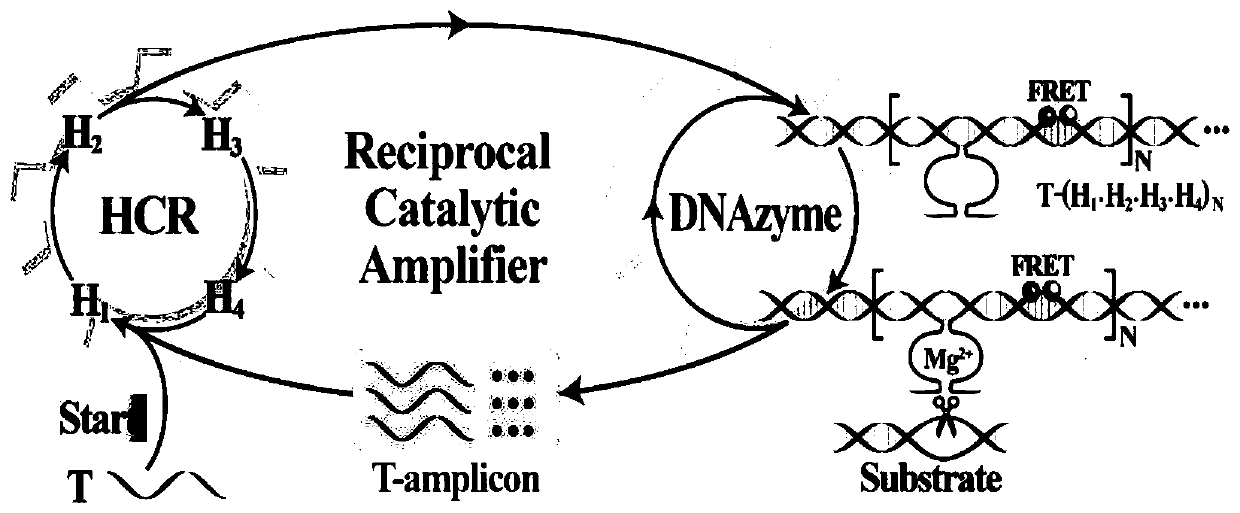
[0035] Fig. 1 (1) is a schematic diagram of a thermostatic cross-catalyzed nuclease reaction for ...
Embodiment 2
[0039] DNA detection based on isothermal cross-catalyzed nuclease reaction
[0040] In hydroxyethylpiperazine ethyl sulfonate buffer (concentration is 10mM, pH 7.2, containing 1M NaCl and 50mM MgCl 2 ), all DNA reactions (H 1 、H 2 、H 3 、H 4 , S and L are 200nM) and different concentrations of priming chain T (0, 1 × 10 -11 , 5×10 -11 , 1×10 -10 , 5×10 -10 , 1×10 -9 , 5×10 -9 , 1×10 -8 , 2.5×10 -8 , 5×10 -8 M) mixing, incubating at room temperature for 3 h, and measuring the fluorescence intensity of the system using a fluorescence spectrometer (excitation voltage 600V, excitation slit 5nm, emission slit 10nm, excitation wavelength 490nm, wavelength scanning range 505-650nm).
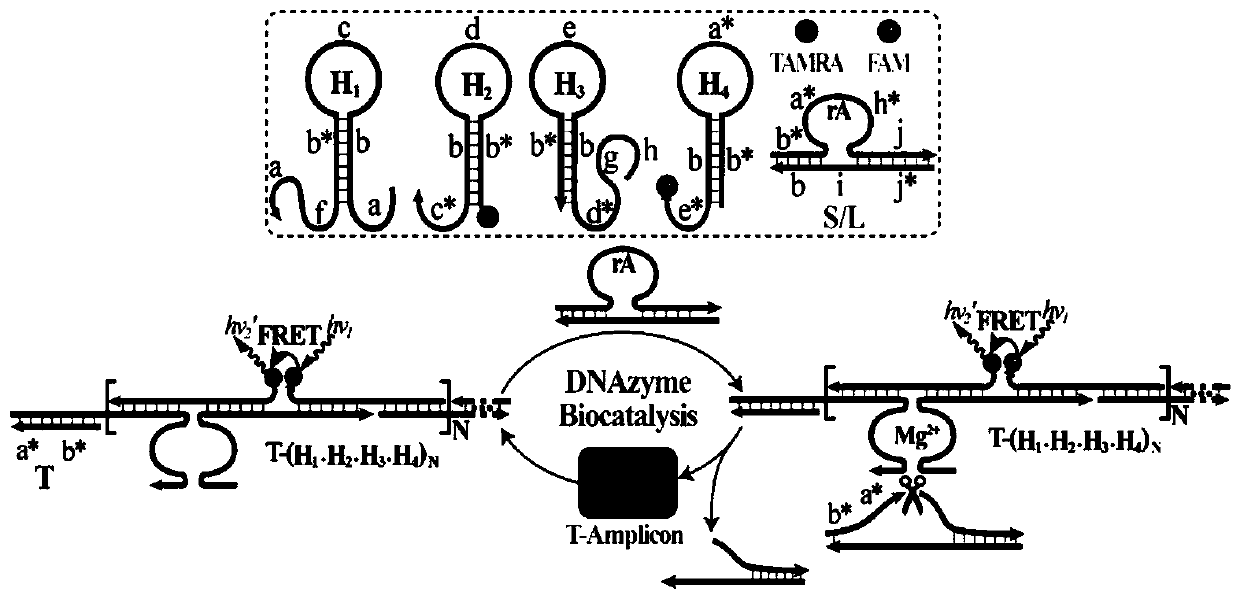
[0041] Depend on image 3 (A) It can be seen that when no target DNA is added to the R-HCR system, each DNA probe can maintain its own stability, and the fluorescence of the system only changes slightly ( image 3 Curve a) in (A), when adding different concentrations of target DNA, the chan...
Embodiment 3
[0045] In vitro detection of miRNA-21 based on isothermal cross-catalyzed nuclease reaction
[0046] In hydroxyethylpiperazine ethyl sulfonate buffer (concentration is 10mM, pH 7.2, containing 1M NaCl and 50mM MgCl 2 ), all DNA reactions (H 1 、H 2 、H 3 、H 4 , S, L are all 200nM, H 5 100nM) with different concentrations of miRNA-21 (0, 1×10 -11 , 5×10 -11 , 1×10 -10 , 5×10 -10 , 1×10 -9 , 5×10 -9 , 1×10 -8 , 3×10 -8 , 5×10 -8 M) mixing, incubating at room temperature for 3 h, and measuring the fluorescence intensity of the system using a fluorescence spectrometer (excitation voltage 600V, excitation slit 5nm, emission slit 10nm, excitation wavelength 490nm, wavelength scanning range 505-650nm).
[0047] Figure 1 (4) is a schematic diagram of the constant temperature cross-catalyzed nuclease reaction for miRNA-21 detection. Depend on Figure 4 (A) It can be seen that when miRNA-21 is not added to the R-HCR system, each DNA probe can maintain its own stability, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com