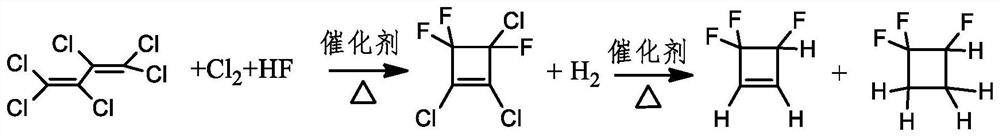
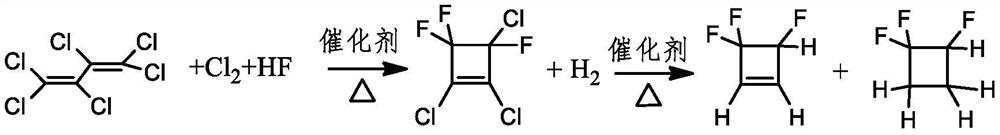
A kind of method of gas phase catalytic synthesis 3,4,4-trifluorocyclobutene
A trifluorocyclobutene, gas phase technology, applied in the field of gas phase catalytic synthesis of 3,4,4-trifluorocyclobutene, can solve the problems of harsh reaction conditions, long technical route, expensive raw materials, etc., and achieve high product yield , the effect of reducing risk and convenient source
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) adopt co-precipitation method to prepare ring fluorination catalyst, the steps are as follows:
[0027] CrCl with a molar ratio of 75:5:20 3 , Co(NO 3 ) 2 , In(NO 3 ) 3 The solutions are mixed, 30 wt% ammonia water is added dropwise to the mixed solution, the pH is adjusted to 9.0, the precipitate is filtered, washed with deionized water, dried, and press-molded to obtain the ring fluorination catalyst precursor Cr-Co-In;
[0028] Put 50ml of ring fluorination catalyst Cr-Co-In precursor into the fixed bed reactor, and the fixed bed reactor is heated by an open tube heating furnace. Under the protection of 100ml / min nitrogen, the catalyst was first dried at a rate of 1°C / min to 400°C for 10 hours, and then the temperature was lowered to 200°C. This completes the drying process of the ring fluorination catalyst.
[0029] Heat the reactor to 300°C, activate the catalyst with 100ml / min nitrogen and 20ml / min hydrogen fluoride for 10 hours; activate the catalyst wi...
Embodiment 2
[0037] (1) adopt co-precipitation method to prepare ring fluorination catalyst, the steps are as follows:
[0038] CrCl with a molar ratio of 80:5:15 3 , Mg(NO 3 ) 2 , Cu(NO 3 ) 2 The solutions were mixed, and 30 wt.% ammonia water was added dropwise to the mixed solution to adjust the pH=9.0. Precipitation is filtered, washed with deionized water, dried, and pressed to obtain ring fluorination catalyst precursor Cr-Mg-Cu.
[0039] Pass 50ml of ring fluorination catalyst Cr-Mg-Cu precursor into the fixed bed reactor, and the fixed bed reactor is heated by an open tube heating furnace. Under the protection of 100ml / min nitrogen, the catalyst was first dried at a rate of 1°C / min to 400°C for 10 hours, and then the temperature was lowered to 200°C. This completes the drying process of the ring fluorination catalyst.
[0040] Heat the reactor to 300°C, activate the catalyst with 100ml / min nitrogen and 20ml / min hydrogen fluoride for 10 hours; activate the catalyst with 100ml...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com