Preparation method of a 25-hydroxy vitamin D3 intermediate
A technology for hydroxyvitamins and intermediates, which is applied in the field of preparation of 25-hydroxyvitamin D3 intermediates, which can solve the problems of high risk of lithium reagents, and achieve the effects of easy process operation, simple operation of the preparation process, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0060] A form of 25-hydroxyvitamin D 3 Process for the preparation of intermediates, the 25-hydroxyvitamin D 3 The intermediate is 2-methyl-4-(phenylsulfonyl)-2-butanol, which is prepared from 3-methyl-3-butanol-1-p-toluenesulfonate, halide and sodium benzenesulfinate in Made in solvent.
[0061] That is, the application provides a novel 25-hydroxyvitamin D 3 Intermediate C 5 The fragment is an alcohol sulfone fragment, which has high safety. The preparation process is simple, easy to separate, and the raw material is easy to obtain. It is 25-hydroxyvitamin D 3 The synthesis of intermediates provides a new, more economical and safer way of thinking.
[0062] Specifically, the present invention provides a two-step and "one-pot" method for synthesizing 25-hydroxyvitamin D 3 Intermediates, wherein the two-step process comprises:
[0063] 1) Under the protection of an inert gas, react 1 mol of 3-methyl-3-butanol-1-p-toluenesulfonate with 1.0-2.5 mol of halide in the first or...
Embodiment 1
[0072] A form of 25-hydroxyvitamin D 3 The preparation method of intermediate comprises the following steps:
[0073] Step 1) Preparation of 4-bromo-2-methyl-2-butanol:
[0074] Under nitrogen protection, add 0.10mol 3-methyl-3-butanol-1-p-toluenesulfonate, 0.15mol sodium bromide and 20mL methanol to a 500mL reaction flask, stir magnetically and heat to reflux, then add 0.05mol DMF (moisture content 1.0%), continue to reflux reaction for 6.0h;
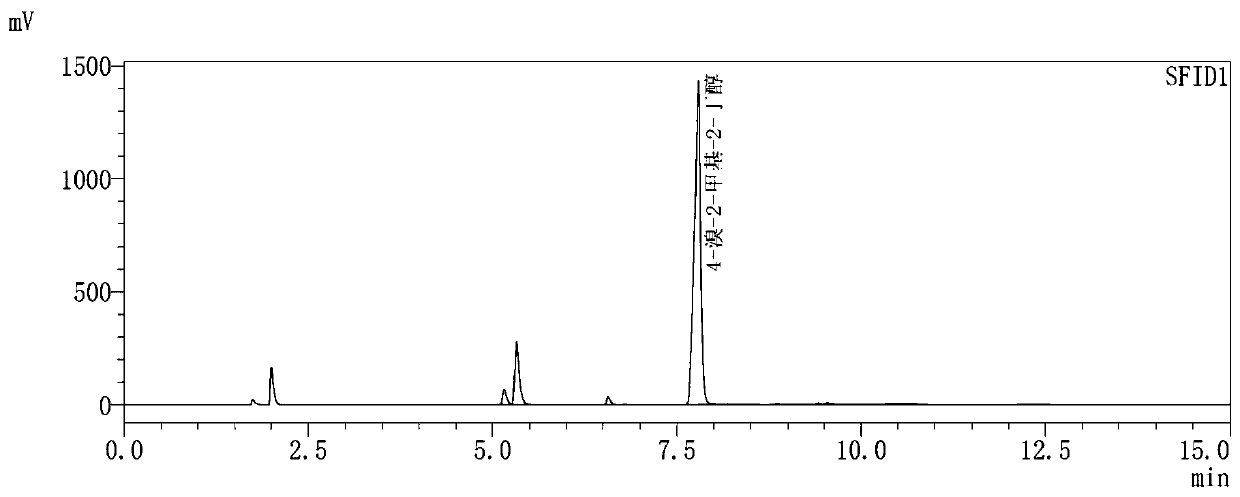
[0075] After the reaction was complete, methanol was recovered under reduced pressure, and 100 mL of water was added to dissolve the solid, extracted three times with dichloromethane, the organic phases were combined, the dichloromethane was recovered, drained, the concentrate was refined under reduced pressure, and the fractions were collected to obtain 16.19 g of a colorless liquid , GC content 82.41%, GC graph as shown in figure 1 As shown, the yield calculated based on 3-methyl-3-butanol-1-p-toluenesulfonate was 79.87%.
[0076...
Embodiment 2
[0080] A form of 25-hydroxyvitamin D 3 The preparation method of intermediate comprises the following steps:
[0081] Step 1) Preparation of 4-bromo-2-methyl-2-butanol:
[0082] Under nitrogen protection, add 0.05mol 3-methyl-3-butanol-1-p-toluenesulfonate, 0.08mol sodium bromide and 20mL acetone to a 500mL reaction flask, stir magnetically and heat to reflux, then add 0.05mol DMF (moisture content 2.5%), continue the reflux reaction for 7.0h;
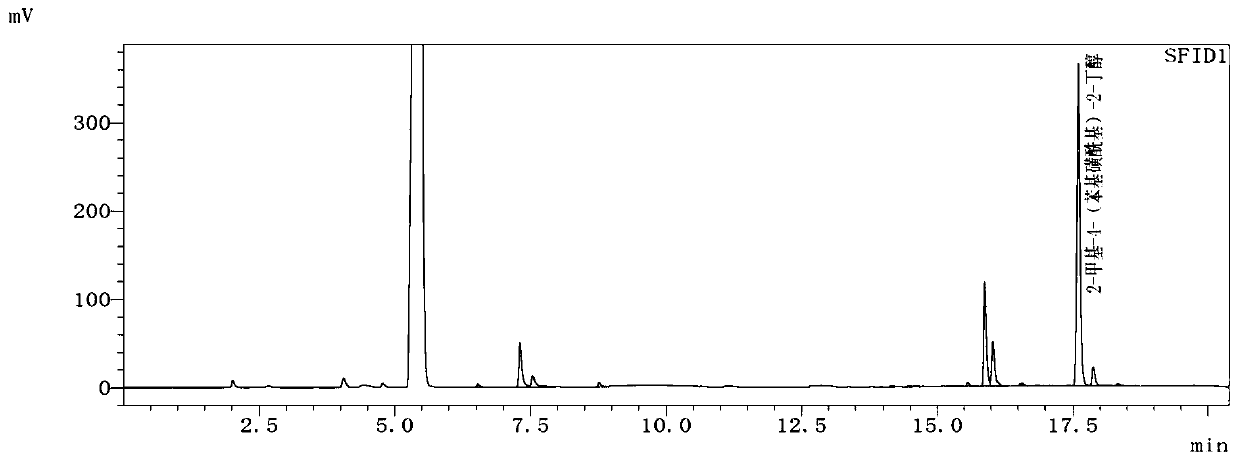
[0083] After the reaction was completed, acetone was recovered under reduced pressure, and 60 mL of water was added to dissolve the solid, extracted three times with dichloromethane, the organic phases were combined, the dichloromethane was recovered, drained, the concentrate was refined under reduced pressure, and 8.10 g of colorless liquid was obtained by collecting fractions , the GC content is 86.86%, and the yield calculated by 3-methyl-3-butanol-1-p-toluenesulfonate is 85.28%.
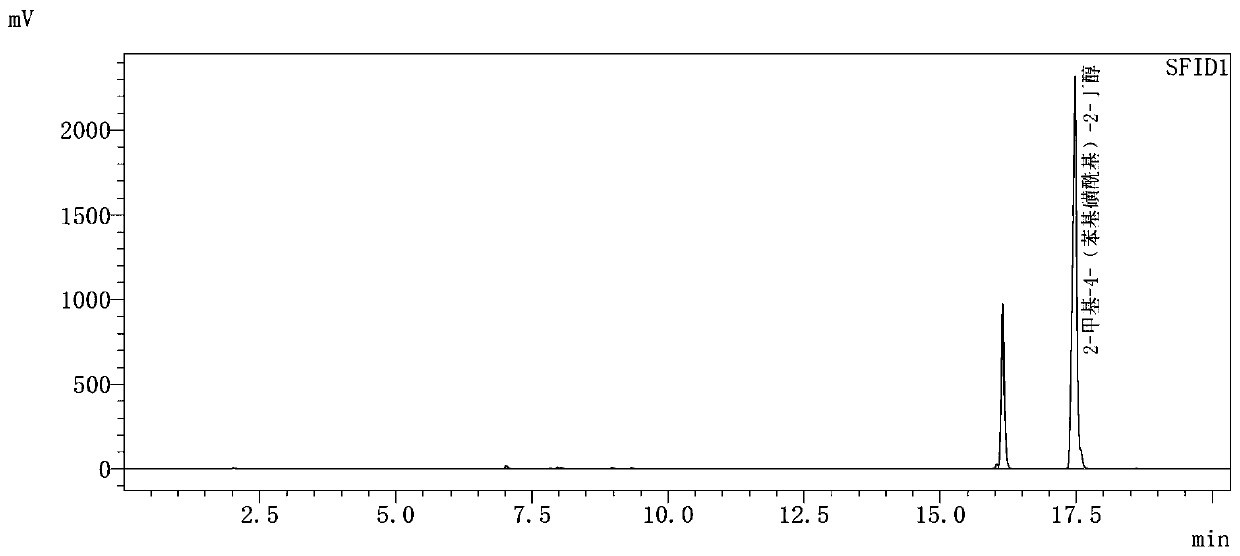
[0084] Step 2) Preparation of 2-methyl-4-(phenyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com