A method for removing arsenic and chlorine from polluted acid wastewater of metallurgical enterprises
A technology for polluted acid wastewater and polluted acid, which is applied in the fields of metallurgical wastewater treatment, water/sewage treatment, chemical instruments and methods, etc., can solve the problems of high silver regeneration recovery rate, complex process treatment, high production cost, and reduce processing time. , the effect of reducing environmental pollution and shortening the response time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
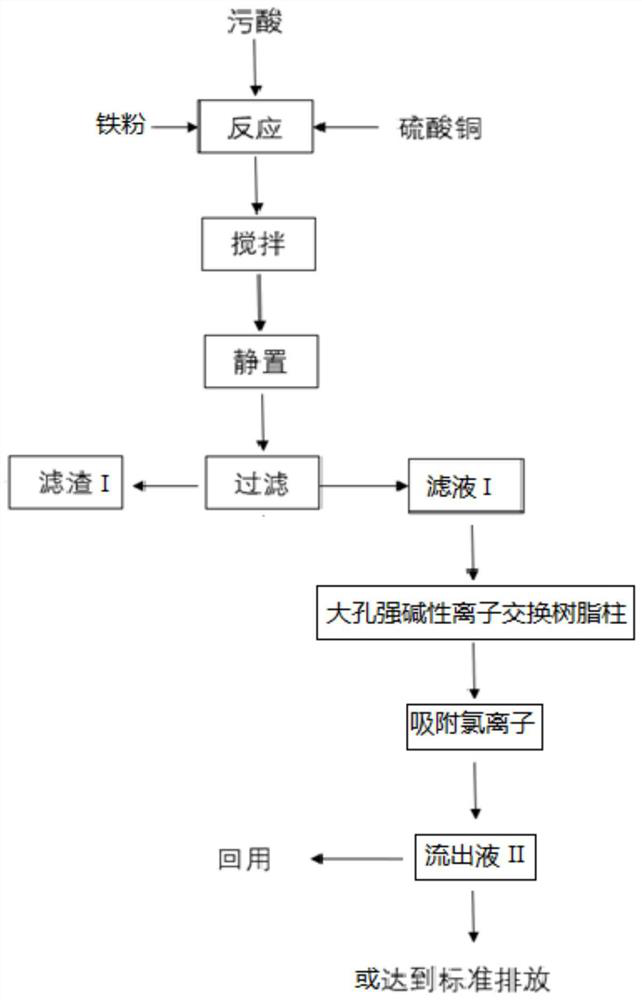
[0137] Take 100mL of dirty acid solution and put it into the reaction kettle, start stirring (100 rpm), and preheat to 35°C, the dirty acid contains As (as H 3 AsO 3 form) 1050mg / L, Cl (as Cl - form) 1300mg / L, Pb (as Pb 2+ form) 116mg / L, Cd (as Cd 2+ form) 101.7mg / L, F (in the form of F - form) 456mg / L, pH=1.2;
[0138] Add 0.432g iron powder, 14.4mL of copper sulfate solution of 50g / L in polluted acid simultaneously, continue to stir (100 revs / min) reaction, the reaction time is 10 minutes, obtains solid-liquid mixture I; Above-mentioned solid-liquid mixture I is pumped Filter, obtain filtrate I and filter residue I, measure the content of arsenic in filtrate I, contain As (with H 3 AsO 3 form) 24.6mg / L; in the above reaction process, the removal rate of arsenic is 97.66%;
[0139] The pretreated strong basic anion exchange resin (calculated as 10g with dry resin) is packed into a column, then the filtrate I is pumped into the column, and the flow rate of the effluent ...
Embodiment 2
[0141] Take 100mL of dirty acid solution and put it into the reaction kettle, start stirring (100 rpm), and preheat to 35°C, the dirty acid contains As (as H 3 AsO 3 form) 1580mg / L, Cl (as Cl - form) 1025mg / L, Pb (as Pb 2+ form) 198mg / L, Cd (as Cd 2+ form) 98mg / L, F (in the form of F - form) 502mg / L, pH=1.0;
[0142] Add 0.648g iron powder, 21.6mL of copper sulfate solution of 50g / L in polluted acid simultaneously, continue to stir (100 revs / min) reaction, the reaction time is 10 minutes, obtains solid-liquid mixture I; Above-mentioned solid-liquid mixture I is pumped Filter, obtain filtrate I and filter residue I, measure the content of arsenic in filtrate I, contain As (with H 3 AsO 3 form) 25.0mg / L; in the above reaction process, the removal rate of arsenic was 97.62%;
[0143] The pretreated strong basic anion exchange resin (calculated as 10g with dry resin) is packed into a column, then the filtrate I is pumped into the column, and the flow rate of the effluent II...
Embodiment 3
[0145] Take 100mL of dirty acid solution and put it into the reaction kettle, start stirring (100 rpm), and preheat to 35°C, the dirty acid contains As (as H 3 AsO 3 form) 1050mg / L, Cl (as Cl - form) 1300mg / L, Pb (as Pb 2+ form) 116mg / L, Cd (as Cd 2+ form) 101.7mg / L, F (in the form of F - form) 456mg / L, pH=1.2;
[0146] Add 0.510g iron powder, 17.0mL of copper sulfate solution of 50g / L in polluted acid simultaneously, continue to stir (100 revs / min) reaction, the reaction time is 10 minutes, obtains solid-liquid mixture I; Above-mentioned solid-liquid mixture I is pumped Filter, obtain filtrate I and filter residue I, measure the content of arsenic in filtrate I, contain As (with H 3 AsO 3 form) 21.3mg / L; in the above reaction process, the removal rate of arsenic is 97.97%;
[0147] The pretreated strong basic anion exchange resin (calculated as 10g with dry resin) is packed into a column, then the filtrate I is pumped into the column, and the flow rate of the effluent ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com