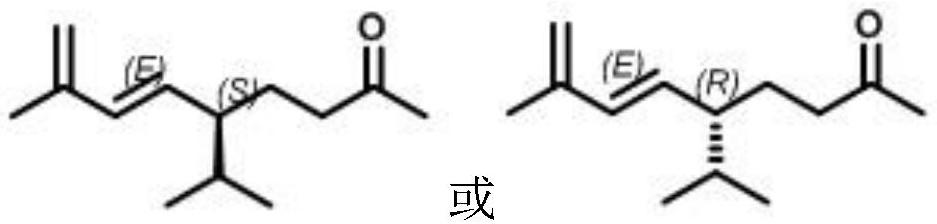
A kind of (e) type solanone, its preparation method and application
A technology of solanone and isopropyl, which is applied in the field of organic synthesis, can solve the problems of long routes, increased safety risks and costs, and achieve the effects of high safety, enhanced aroma elegance, and enhanced smoke fullness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
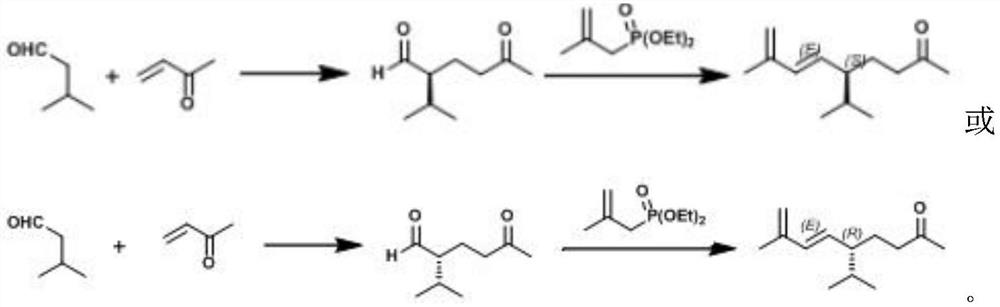
[0026] Embodiment 1: the synthesis of (S)-2-isopropyl-5-carbonyl hexanal
[0027] Place 73mg (about 0.4mmol) of ethyl 3,4-dihydroxybenzoate in a round bottom flask, and add 53.48mg (0.2mmol) of (S)-2-(methoxybenzhydryl)pyrrole in sequence alkanes, 215 μL (approximately 2 mmol) of isovaleraldehyde and 243 μL (approximately 3 mmol) of methyl vinyl ketone, the reaction was stirred at room temperature, the solid dissolved, and stirred for 24 hours.
[0028] After the reaction was finished, the remaining methyl vinyl ketone was removed by rotary evaporation, and the remaining liquid was separated by column chromatography of sherwood oil: ethyl acetate=20:1 to obtain 230.1 mg of a light yellow liquid, with a yield of 74%; analyzed as (S )-2-isopropyl-5-carbonylhexanal. analyse as below:
[0029] 1 H NMR (400MHz, Chloroform-d) δ9.59((d, J=2.8Hz, 1H), 2.47(m, 1H), 2.35(m, 1H), 2.11(s, 3H), 2.07-1.99(m , 2H), 1.85-1.70 (m, 2H), 0.98 (d, J=6.6Hz, 3H), 0.95 (d, J=6.6Hz, 3H). 13 C NM...
Embodiment 2
[0032] Embodiment 2: the synthesis of (E)-solanone
[0033] Put 5.2 g (about 27.3 mmol) of (2-methylallyl) diethyl phosphonate in the reaction flask, add 70 mL of methyl tert-butyl ether to dissolve, and 9.5 mL (about 54.6 mmol) of hexamethyl Phosphoric triamide, the system was cooled to minus 78°C; 13.7mL of 2M (about 27.3mmol) sodium hexamethyldisilazide tetrahydrofuran solution was added dropwise, and stirred at minus 78°C for 30 minutes, Finally, a solution of 3.3 g (about 21 mmol) of (S)-2-isopropyl-5-carbonyl hexanal obtained in Example 1 in 70 mL of methyl tert-butyl ether was added, and the temperature was slowly raised to room temperature within 6 hours , and stirred for another 10 hours at room temperature.
[0034] After the reaction, 100 mL of water was added to the system, extracted three times with ether, the combined organic phases were dried over anhydrous sodium sulfate, concentrated, and the remaining liquid was subjected to column chromatography with petrol...
Embodiment 3
[0038] Embodiment 3: the application of (E) type solanone in cigarette shredded tobacco flavoring
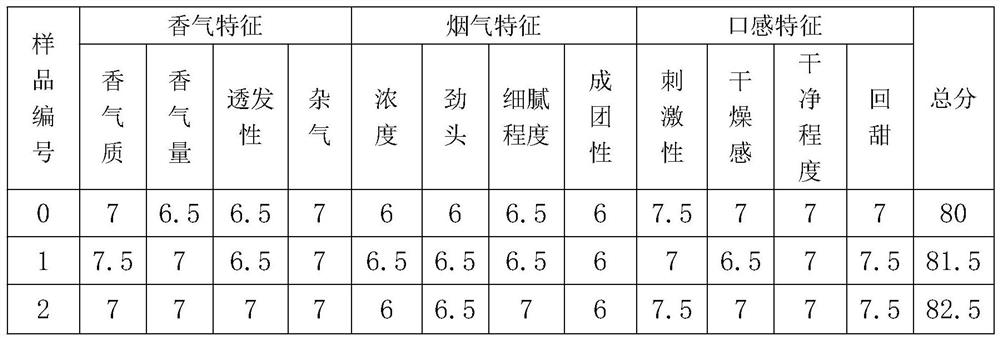
[0039] Taking a certain Yunproduct brand sample cigarette as the experimental object, the added amount of spices was calculated according to the weight of shredded tobacco, and two pure (E) type solanones synthesized in Example 2 of the present invention: (S, E)-5-isopropyl- 8-Methyl-6,8-dien-2-one (injection plus cigarette as sample 1) and (R,E)-5-isopropyl-8-methyl-6,8-diene-2 -ketone (injection plus cigarettes is sample 2) is made into ethanol solution of a certain concentration, and with CIJECTOR flavor and fragrance injection machine, its ethanol solution is evenly injected into cigarettes according to the percentage of spices in tobacco shreds by 0.005% by weight; inject under the same conditions The cigarette branch (sample 0) of same volume ethanol is control sample, at relative humidity 60%, 22 ℃ of conditions balance 48 hours, according to tobacco industry standard " t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com