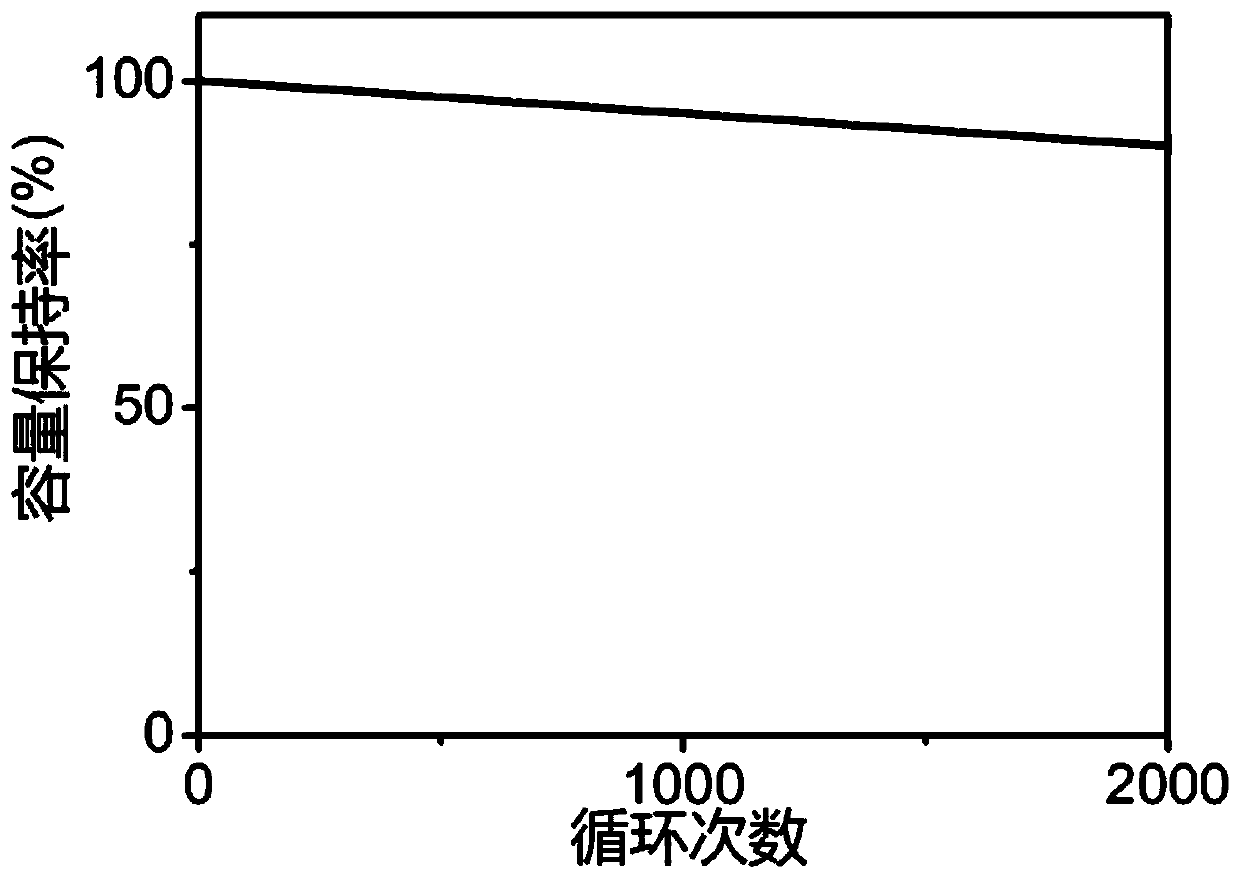
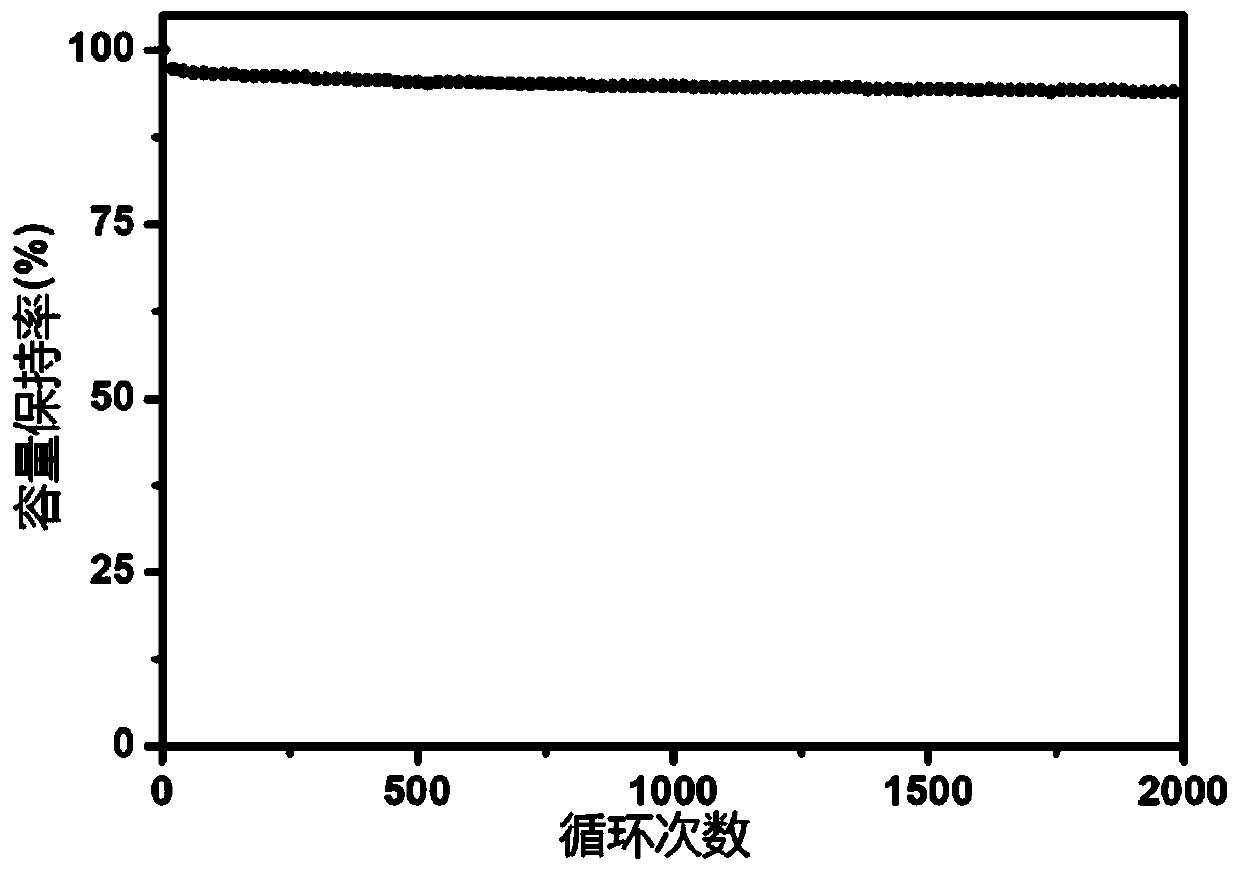
Lithium battery
A lithium battery and electrolyte technology, applied in secondary batteries, battery electrodes, secondary battery manufacturing, etc., can solve the problems of flammability of organic solvents, poor safety, and high production costs, and achieve a wide operating temperature range and high voltage window. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The specific composition of the water-based electrolyte in this example is solvent water, the functional solute for limiting water molecules is sucrose, and the electrolyte is lithium perchlorate. The preparation method is as follows: 2 g of sucrose and 0.17 g of lithium perchlorate are successively added to Put it in a glass sample bottle of deionized water, seal it, put it in a blast drying oven at 90°C and heat it for 20 minutes until it is fully dissolved, and then obtain the aqueous electrolyte solution of this example after cooling. Among them, the mass fraction of sucrose in the electrolyte is 63.1%. The electrochemical window of the aqueous electrolyte prepared in this example was tested by linear voltammetry (saturated calomel electrode as reference electrode, platinum disk electrode as working electrode, and platinum plate electrode as counter electrode). The results show that the electrochemical stability window of the aqueous electrolyte is 2.7V, and the aqu...
Embodiment 2
[0036] The specific composition of the water-based electrolyte in this example is solvent water, the functional solute of restricted water molecules is fructose, and the electrolyte is lithium perchlorate. Put it in a glass sample bottle of deionized water, seal it, put it in a blast drying oven at 90°C and heat it for 20 minutes until it is fully dissolved, and then obtain the aqueous electrolyte solution of this example after cooling. Among them, the mass fraction of fructose in the electrolyte is 63.1%. The electrochemical window of the aqueous electrolyte prepared in this example was tested by linear voltammetry (saturated calomel electrode as reference electrode, platinum disk electrode as working electrode, and platinum plate electrode as counter electrode). The results show that the electrochemical stability window of the aqueous electrolyte is 2.6V, and the aqueous electrolyte will not freeze at -40°C, and there is no solute precipitation.
[0037] The aqueous electro...
Embodiment 3
[0039] The specific composition of the water-based electrolyte in this example is solvent water, the functional solute of restricted water molecules is maltose, and the electrolyte is lithium perchlorate. The preparation method is as follows: Weigh 1.5g of maltose and 0.17g of lithium perchlorate into a Put 1g of deionized water in a glass sample bottle, seal it, put it in a blast drying oven at 90°C and heat it for 20 minutes until fully dissolved, and then obtain the aqueous electrolyte solution of this example after cooling. Among them, the mass fraction of maltose in the electrolyte is 56.2%. The electrochemical window of the aqueous electrolyte prepared in this example was tested by linear voltammetry (saturated calomel electrode as reference electrode, platinum disk electrode as working electrode, and platinum plate electrode as counter electrode). The results show that the electrochemical stability window of the aqueous electrolyte is 2.5V.
[0040] The aqueous electro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Discharge capacity | aaaaa | aaaaa |
| Discharge capacity | aaaaa | aaaaa |
| Discharge capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com