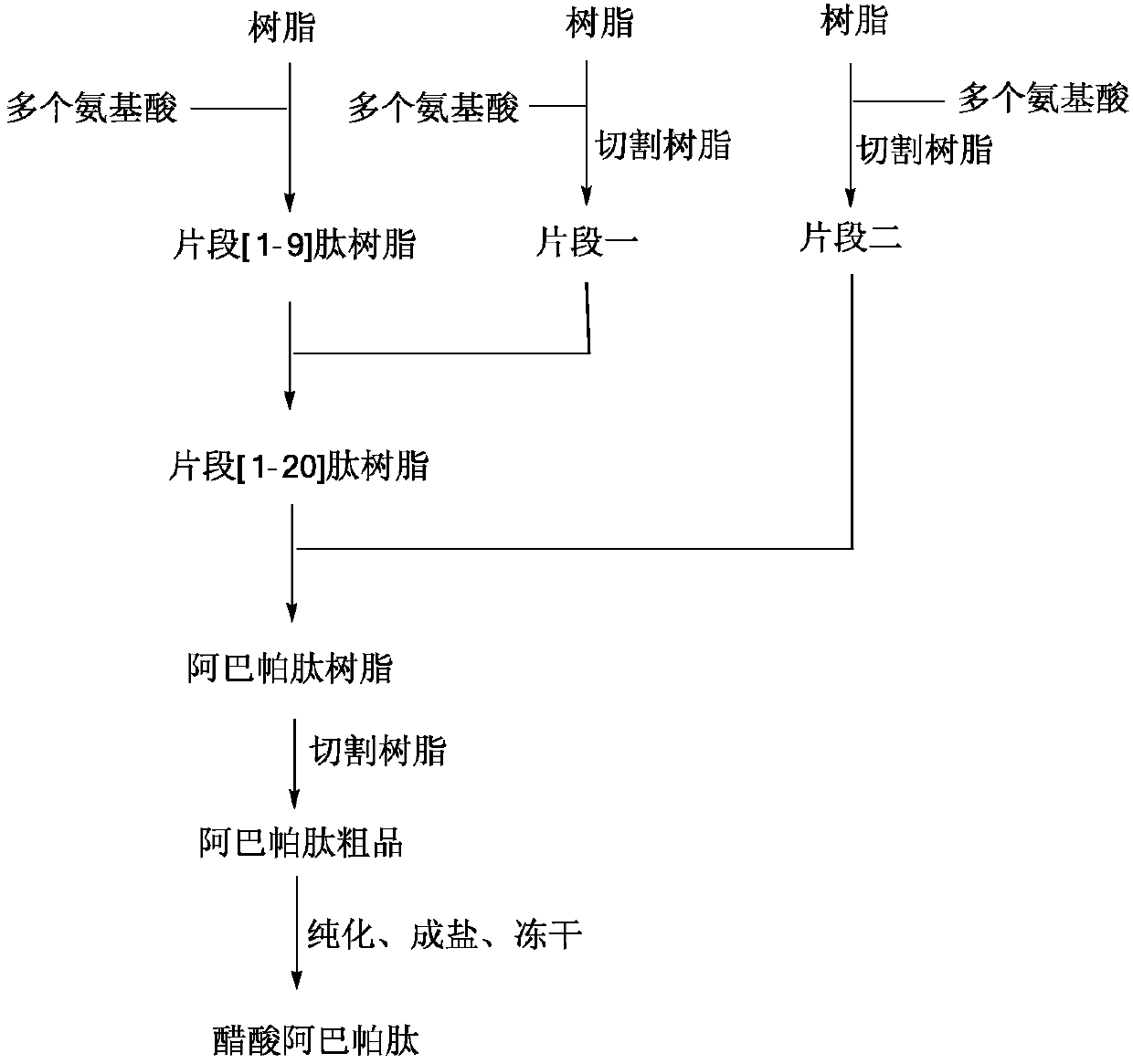
Solid-phase method of Abaloparatide
A synthetic method, the technology of abaloparatide, applied in the field of solid phase synthesis technology to realize the synthesis process of abaloparatide, can solve the problems of many impurities, excessive waste liquid, cumbersome operation steps, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1: Synthesis of Fragment [1-9] Peptide Resin
[0061]Add Rink-Amide-MBHA Resin (15.00g, 4.25mmol) and 200mL DCM in turn to the solid-phase reaction bottle, and suction filter after swelling. The filter cake was mixed with 300mL of 20% Pip / DMF solution, stirred for 5 minutes, and suction filtered to obtain the filter cake; then 300mL of 20% Pip / DMF solution was added, stirred for 15 minutes, and the filter cake was obtained by suction filtration. Wash with an appropriate amount of DMF, and filter with suction to obtain a filter cake. Add 300mL DMF to Pip / DMF and stir, then add Fmoc-Ala-OH (4.20g), HOBT (1.82g) and DIC (1.70g) sequentially within 30min under ice bath. Stir for about 2 h under nitrogen protection until the reaction is detected by ninhydrin. Wash with an appropriate amount of DMF and drain. Repeat the above deprotection and coupling reaction steps, and connect the amino acids Fmoc-Thr(tBu)-OH, Fmoc-His(Trt)-OH, Fmoc-Leu-OH, Fmoc-Lys(Boc)-OH, Fmoc...
Embodiment 2
[0063] Embodiment two: the synthesis of fragment one
[0064] Add 2-CTC Resin (25.00g) and 400mL DCM to the solid-phase reaction flask in sequence, and suction filter after swelling. Add Fmoc-Glu(OtBu)-OH (8.51g) and 100mL DCM, add DIEA (about 9.53g) dropwise, stir for 2 hours with nitrogen bubbling, then add MeOH (25mL), stir for 30min and filter with suction, filter cake through DMF, DCM was alternately washed and dried in vacuo.
[0065] The above-mentioned materials and 200mL DCM were sequentially added into the solid-phase reaction bottle, and suction filtered after swelling. The filter cake was mixed with 100mL of 20% Pip / DMF solution, stirred for 5 minutes, and suction filtered to obtain the filter cake; then 100mL of 20% Pip / DMF solution was added, stirred for 15 minutes, and suction filtered. The filter cake was washed with an appropriate amount of DMF, and the filter cake was obtained by suction filtration. Pip / DMF. Add 400 mL of DMF, stir, and add Fmoc-Leu-OH (1...
Embodiment 3
[0067] Embodiment three: the synthesis of fragment two
[0068] Add 2-CTC Resin (25.00g) and 400mL DCM into the solid-phase reaction bottle, and suction filter after swelling. Fmoc-Ser(tBu)-OH (6.88g) and 100mL DCM were added, DIEA (about 9.53g) was added dropwise under nitrogen bubbling, stirred for 2h, then MeOH (25mL) was added, stirred for 30min. After filtering, the filter cake was alternately washed three times with MeOH and DCM, and dried under vacuum at room temperature. After testing, the degree of substitution is 0.74mmol / g;
[0069] Add the dry above-mentioned resin and 200mL DCM to the solid-phase reaction bottle, swell, and filter with suction. The filter cake was mixed with 100mL of 20% Pip / DMF solution, stirred for 5 minutes, and suction filtered to obtain the filter cake; then 100mL of 20% Pip / DMF solution was added, stirred for 15 minutes, and suction filtered. The filter cake was washed with an appropriate amount of DMF and drained. Add 400 mL of DMF and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com