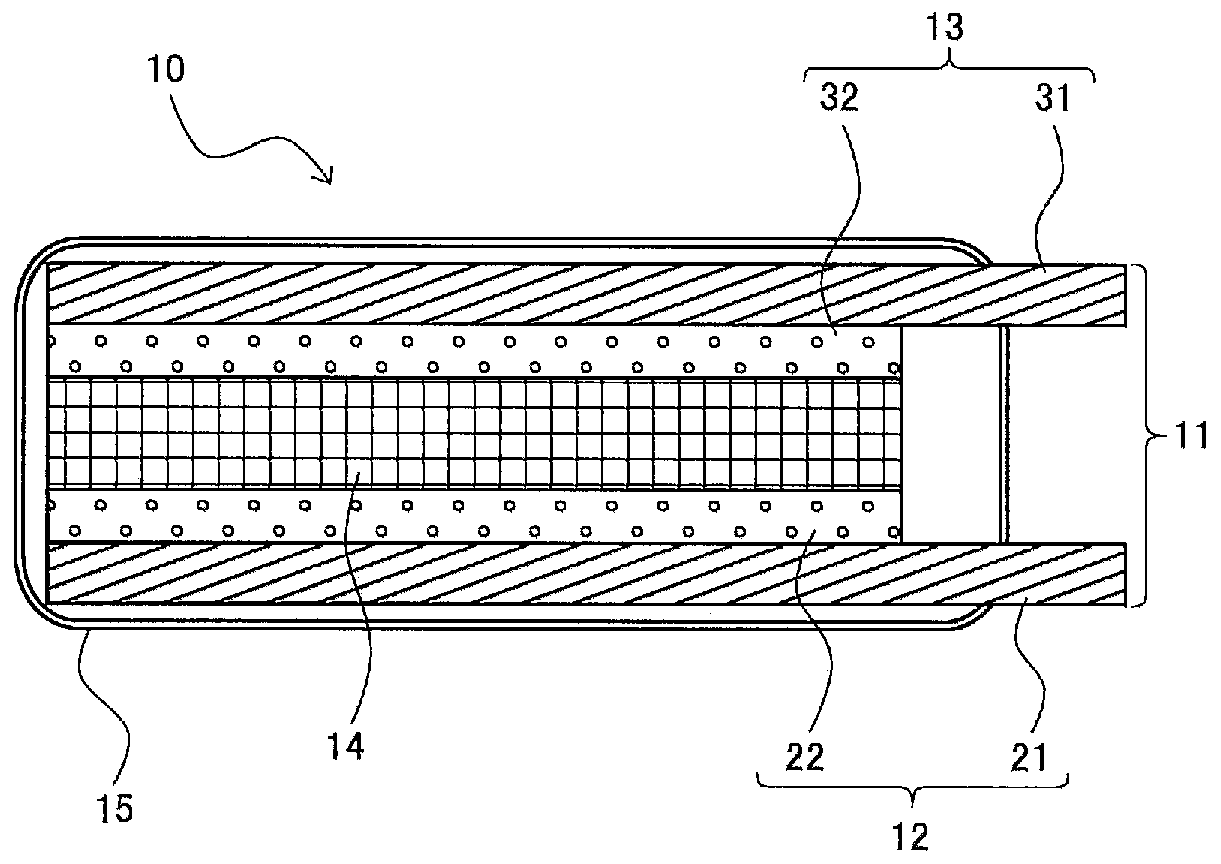
Gel electrolyte, hard gel electrolyte, and electrochemical device
A gel electrolyte and electrochemical technology, which is applied in the direction of electrolyte immobilization/gelation, non-aqueous electrolyte storage battery, electrochemical generator, etc., can solve problems such as undeniable electrolyte leakage, and achieve good device performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
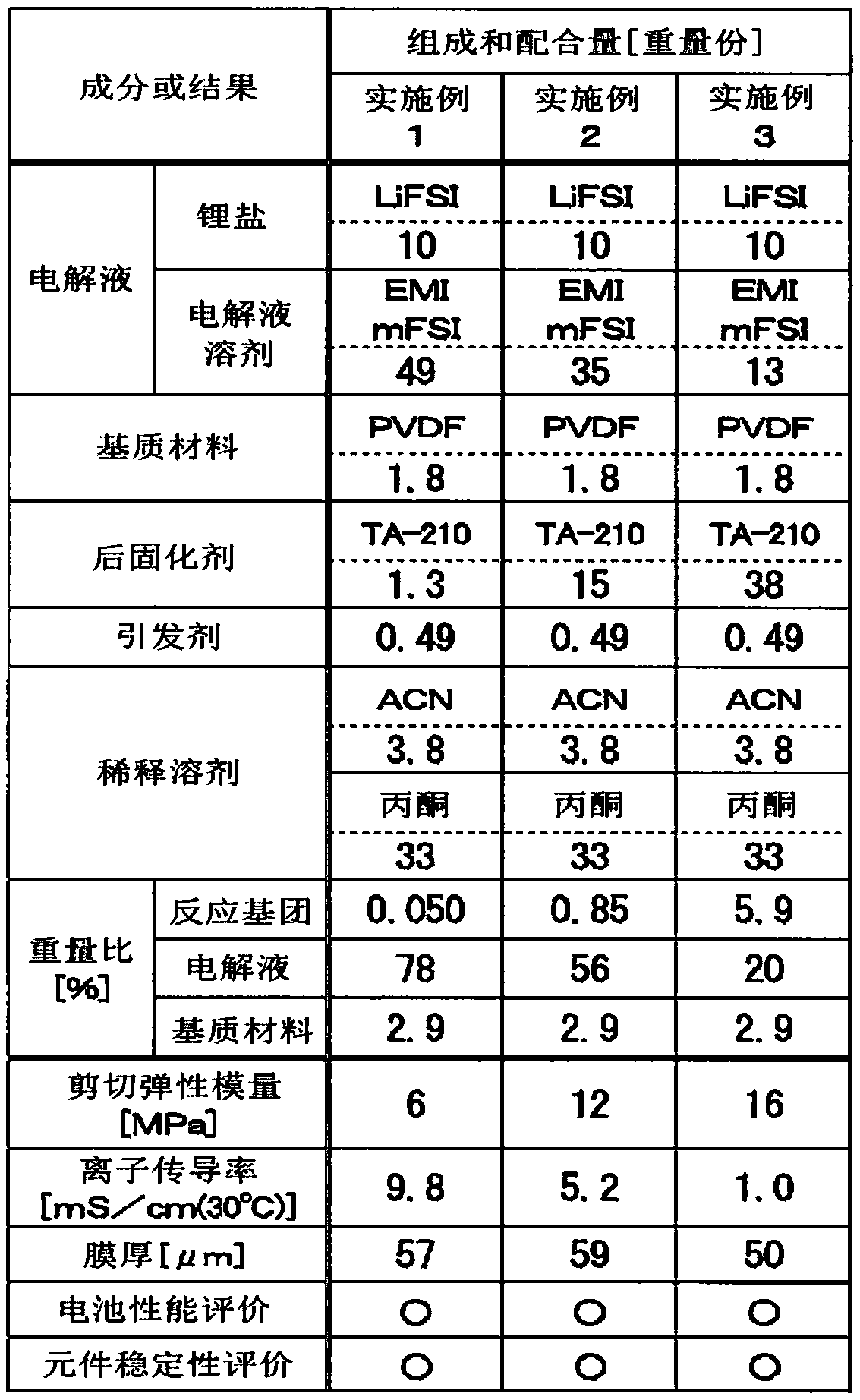
[0135] The following work is carried out in a dry air atmosphere with a dew point below -50°C. As shown in Table 1, 1.8 parts by mass of polyvinylidene fluoride (PVDF, manufactured by KUREHA Co., Ltd., product name: KF POLYMER#7200) as a matrix material was mixed with acetone (Wako Pure Chemical Industries, Ltd. (manufactured) 33 parts by mass were heated and dissolved at 80° C., and left still at room temperature to prepare a PVDF / acetone gel (non-electrolyte gel-like body).
[0136] In addition, as shown in Table 1, 10 parts by mass of lithium bis(fluorosulfonyl)imide (LiFSI, manufactured by KISHIDA Chemical Co., Ltd., lithium battery grade (LBG)) as a lithium salt was used as an electrolyte solution of an ionic liquid system. As a solvent, 49 parts by mass of 1-ethyl-3-methylimidazolium bis(fluorosulfonyl)imide (EMImFSI, manufactured by Daiichi Kogyo Pharmaceutical Co., Ltd., product name: Elexcel IL-110) as a post-curing agent 1.3 parts by mass of tetrafunctional polyethe...
Embodiment 2、3
[0145]Except that the compounding amount of the electrolyte solvent and the compounding amount of the post-curing agent were changed as shown in Table 1, the gel electrolyte related to Example 2 or the gel related to Example 3 were produced in the same manner as in Example 1. electrolyte, and a coin cell for evaluation as the electrochemical device according to Example 2 or the electrochemical device according to Example 3 was produced.
[0146] It should be noted that, as shown in Table 1, in the gel electrolyte involved in Example 2 or the gel electrolyte involved in Example 3, the mass ratio of the reactive group relative to the mass of the electrolyte solvent is between 0.03 and 6.5 mass %, the mass ratio of the electrolyte solvent to the total mass is in the range of 20-80 mass%, and the mass ratio of the matrix material to the total mass is in the range of 1.0-10 mass%.
[0147] For the obtained gel electrolyte related to Example 2 or 3, as described above, the shear ela...
Embodiment 4
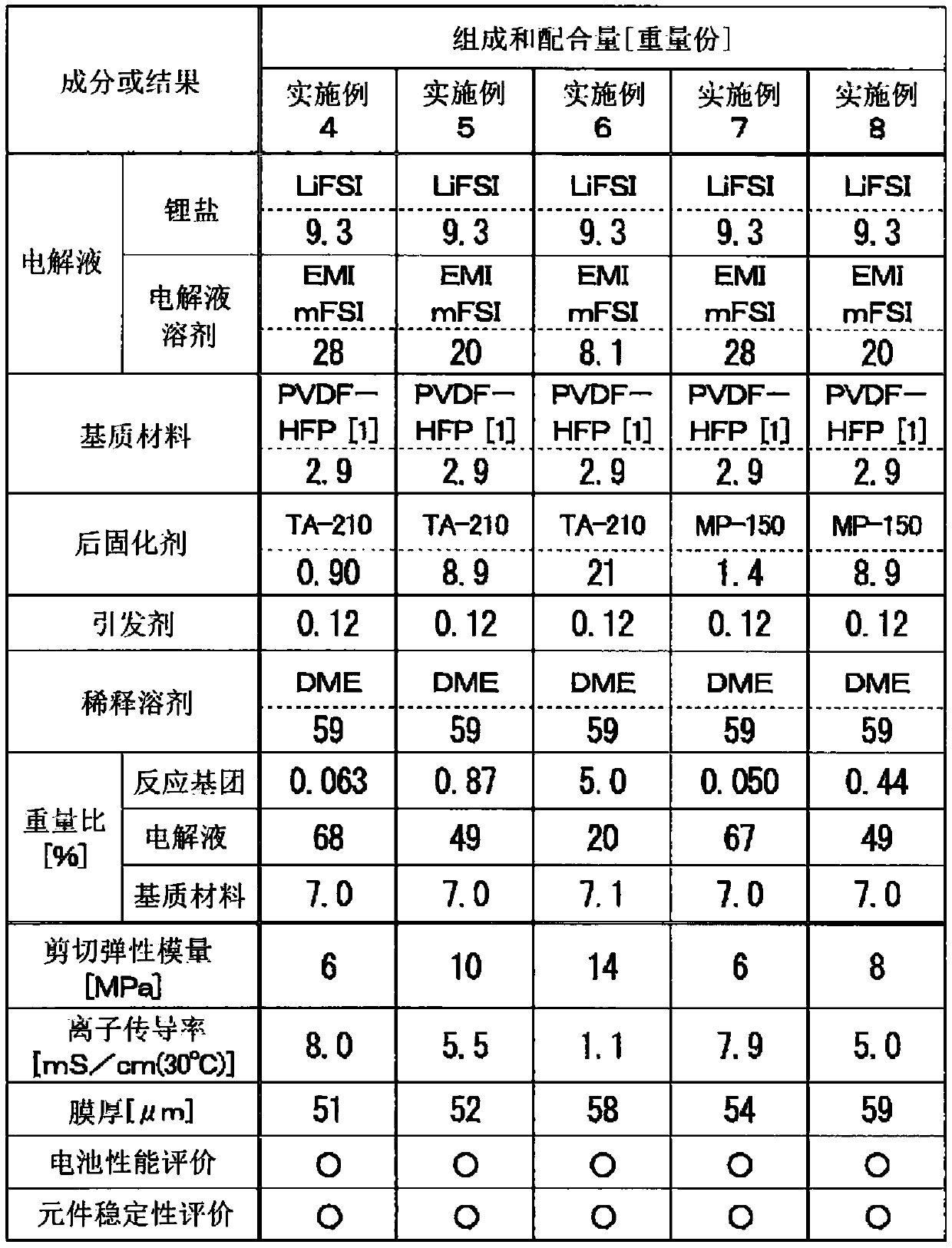
[0151] The following operations were carried out in a dry air atmosphere having a dew point of -50° C. or lower in the same manner as in Examples 1 to 3. As shown in Table 2, the vinylidene fluoride-hexafluoropropylene copolymer (PVDF-HFP, manufactured by KUREHA Co., Ltd., product name: KFPOLYMER#8500) as a matrix material is abbreviated as "PVDF-HFP [1] in Table 2. ”) 2.9 parts by mass were dissolved by heating at 80° C. with respect to 29.5 parts by mass of dimethyl ether (DME, manufactured by Wako Pure Chemical Industries, Ltd.) as a diluting solvent. Based on this, liquid A was prepared as a DME diluted solution of PVDF-HFP.
[0152] In addition, as shown in Table 2, 9.3 parts by mass of LiFSI (refer to Example 1) as a lithium salt, 28 parts by mass of EMImFSI (refer to Example 1) as an electrolyte solution solvent of an ionic liquid system, and LiFSI (refer to Example 1) as a post-curing agent 0.90 parts by mass of functional polyether acrylate (refer to Example 1), 0.12...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com