Method of inhibiting hydrochloric acid from generating during recycling of waste plastic
A technology of recycling and waste plastics, applied in plastic recycling, products, reagents, etc., can solve problems such as loss of function of stabilizers, rusting of manufacturing machinery, etc., achieve the effect of reducing storage area, improving mechanical strength, and achieving high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Hereinafter, an embodiment of the present invention having the above configuration will be described. The evaluation of the acidity test was performed as follows.
[0039] Magnesium oxide (MgO ) 1% by weight, 0.1% by weight, and 0.01% by weight were mixed with a drum to prepare three kinds of sample materials. In addition, CD and polypropylene (PP) without adding an acid acceptor were prepared as comparative materials. Next, each of the aforementioned sample materials and comparative materials was molded at 220° C. using a 50 t injection molding machine to prepare five types of test samples.
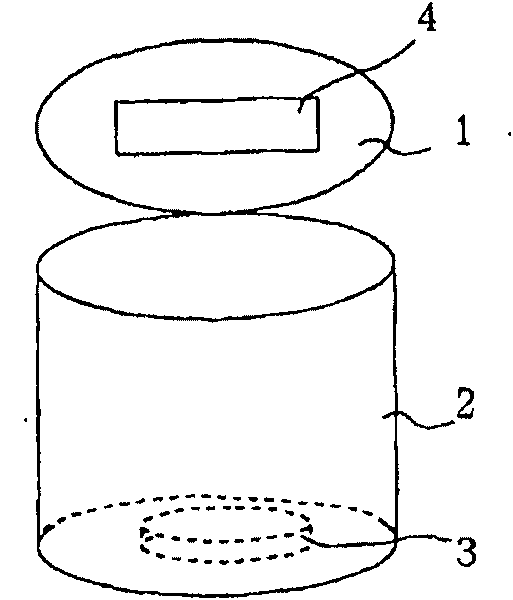
[0040] The acidity of the above samples was measured. Such as figure 1 As shown, for the can main body 2 with the opening and closing lid 1, the aforementioned injected sample 3 was immediately put into the can main body 2, the lid 1 was closed, and left at room temperature for more than 8 hours. A reagent 4 for measuring pH such as litmus paper is pasted on the inside of the...
Embodiment 2
[0046] Hereinafter, in order to measure the acidity more accurately, a case where ion chromatography is used will be described. The acid acceptor is generally in the form of a powder. Therefore, various powders of magnesium oxide, hydrotalcite, calcium carbonate, magnesium carbonate, calcium hydroxide, and magnesium silicate are used as the acid acceptor.
[0047] However, when tumble mixing is performed in a powder state, the dispersibility is poor due to high powder aggregation. In addition, the bulk specific gravity of the powder is small and the volume is large, so it is not good for recycling plastic materials. CDs are mixed with various materials such as low-density polyethylene (LDPE), polypropylene (PP), polyethylene terephthalate (PET), nylon, and polyvinyl chloride (PVC).
[0048] That is, materials with different melting points are mixed in the CD. PVC is included in these mixed materials, and PVC is considered to be the cause of hydrochloric acid generation. Co...
Embodiment 3
[0067] In embodiment 3, adopt correct method to carry out the mensuration of hydrochloric acid again. In this example, evaluation was performed on the case of using magnesia powder and hydrotalcite powder alone as the acid acceptor and the case of using them in combination.
[0068] (Evaluation sample preparation method)
[0069] First, as the raw material before granulation, prepare the loose material for cleaning and centrifugal dehydration, and use a drying oven at 50°C to dry the raw material for 10 minutes, the raw material for 20 minutes, and the raw material for 30 minutes. The acid acceptor is used alone to oxidize Magnesium powder, hydrotalcite powder and mixtures of these are used, and the base resin is made of low-density polyethylene (LDPE) as a masterbatch.
[0070] (test methods)
[0071] The aforementioned dried raw materials were put into a twin-screw extruder (diameter 50 mm, co-rotating twin-screw type, L / D-28), and the concentration of hydrochloric acid in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com