Preparation method of N-(3-hydroxypropyl)phthalimide and catalyst for method
A technology of catalyst and phthalimide, which is applied in the field of preparation of fine chemicals, can solve the problems of high cost and high catalyst toxicity, and achieve the effects of high yield, low catalyst cost and reduced production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Preparation of catalyst:
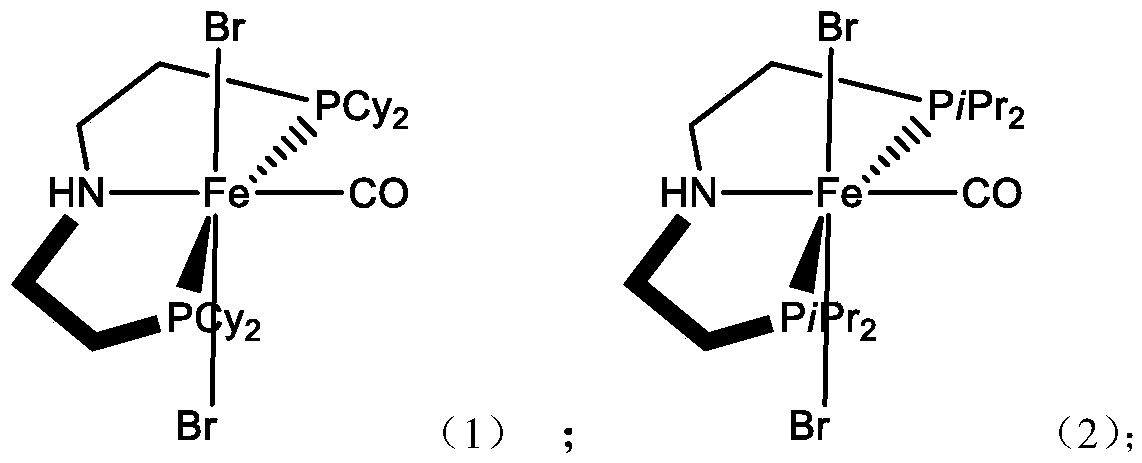
[0043] Under nitrogen atmosphere, add 216.0g (1mol) FeBr 2 Dissolve 280.0g (5mol) Fe powder in 108.1g (1.5mol) tetrahydrofuran and react for 3 hours, and remove the solvent by distillation under reduced pressure to obtain off-white 342.5g (0.95mol) FeBr 2 (THF) 2 Precipitation; further solid 497.5 (0.95mol) HN (CH 2 CH 2 PCy 2 ) 2 Dissolve in 100mL CH 3 Add dropwise to 342.5 (0.95mol) FeBr after CN 2 (THF) 2, refluxed for 4 hours, and further reacted at room temperature for 5 hours; the obtained solution was frozen with liquid nitrogen, passed through carbon monoxide, reacted at room temperature, and the reaction was stopped when the color of the solution changed to dark purple, and the solvent was distilled off under reduced pressure to obtain Purple solid; further recrystallized in dichloromethane as a solvent to obtain pure iron-based chelate catalyst 1, 667.4 g (0.94 mol).
[0044] Elemental Analysis: C 49.05%Fe 7.92%P 8.71%Br 22....
Embodiment 2
[0048] Preparation of catalyst:
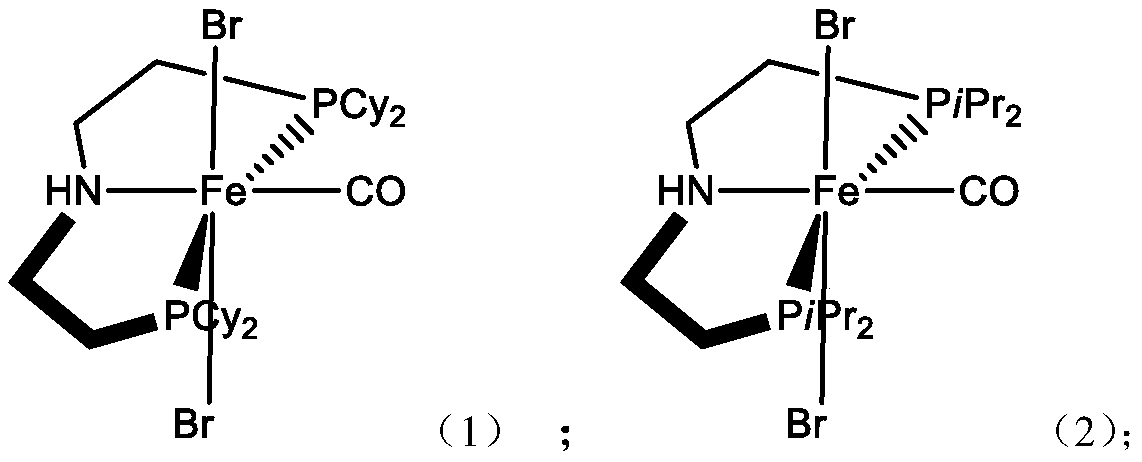
[0049] Except using HN (CH 2 CH 2 PiPr 2 ) 2 instead of HN (CH 2 CH 2 PCy 2 ) 2 Other than that, the preparation process was the same as that of Example 1, and finally, 510.57 g (0.93 mol) of iron-based chelate catalyst 2 was obtained.
[0050] Elemental analysis: C 37.16% Fe 10.20% P 11.30% Br 29.14% O 2.91% H6.74% N2.55%.
[0051] 54.9g (0.1mol) iron-based chelate catalyst 2, the mass fraction of 15% NaBH (CH 3 COO) 3 422.1 g of aqueous solution and 1.5 L of toluene were mixed and stirred for 20 min to form a red homogeneous solution. Then 1471.3 g (10 mol) of phthalimide, 2323.2 g (40 mol) of allyl alcohol, and a mass fraction of 20% K 3 CO 3 885.0g of the aqueous solution was added to a 10L reactor, stirred for 5min and mixed evenly, and then replaced with nitrogen for 3 times, then the oil bath was heated to 80 ° C and normal pressure for 24 hours, after the reaction was completed, it was returned to room temperature, and the ...
Embodiment 3
[0054] 71.0 g (0.1 mol) of iron-based chelate catalyst 1 prepared in Example 1, with a mass fraction of 15% NaBH (CH 3 COO) 3 422.1 g of aqueous solution and 2 L of cyclohexane were mixed and stirred for 20 min to form a red homogeneous solution. Then 1471.3 g (10 mol) of phthalimide, 2904.0 g (50 mol) of allyl alcohol, and a mass fraction of 20% K 3 CO 3 885.0g of the aqueous solution was added to a 10L reactor, stirred for 5min and mixed evenly, and then replaced with nitrogen for 3 times, then the oil bath was heated to 80 ° C and normal pressure for 24 hours, after the reaction was completed, it was returned to room temperature, and the organic matter was extracted with dichloromethane. phase, the dichloromethane was distilled off under reduced pressure, and 1854.82 g of the product was obtained after drying, with a conversion rate of 99.0% and a selectivity of 98.0%.
[0055] Elemental analysis: C 64.42% H 5.32% N 6.91% O 23.35%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com