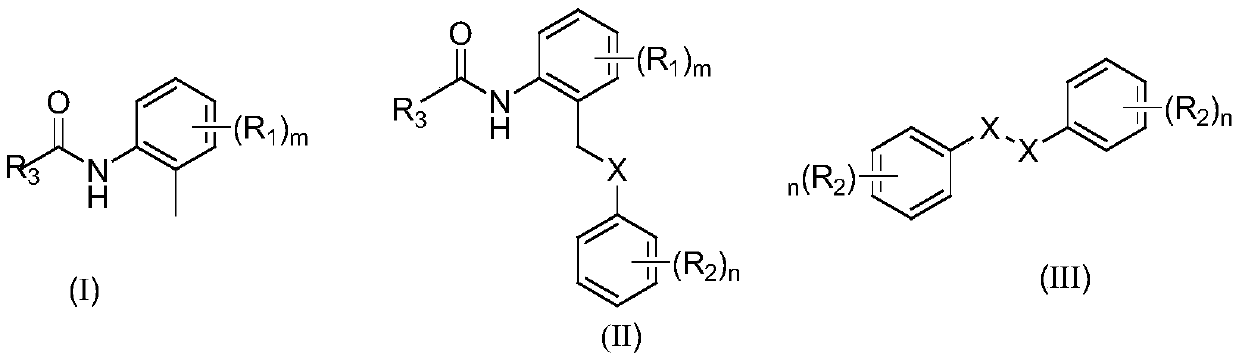
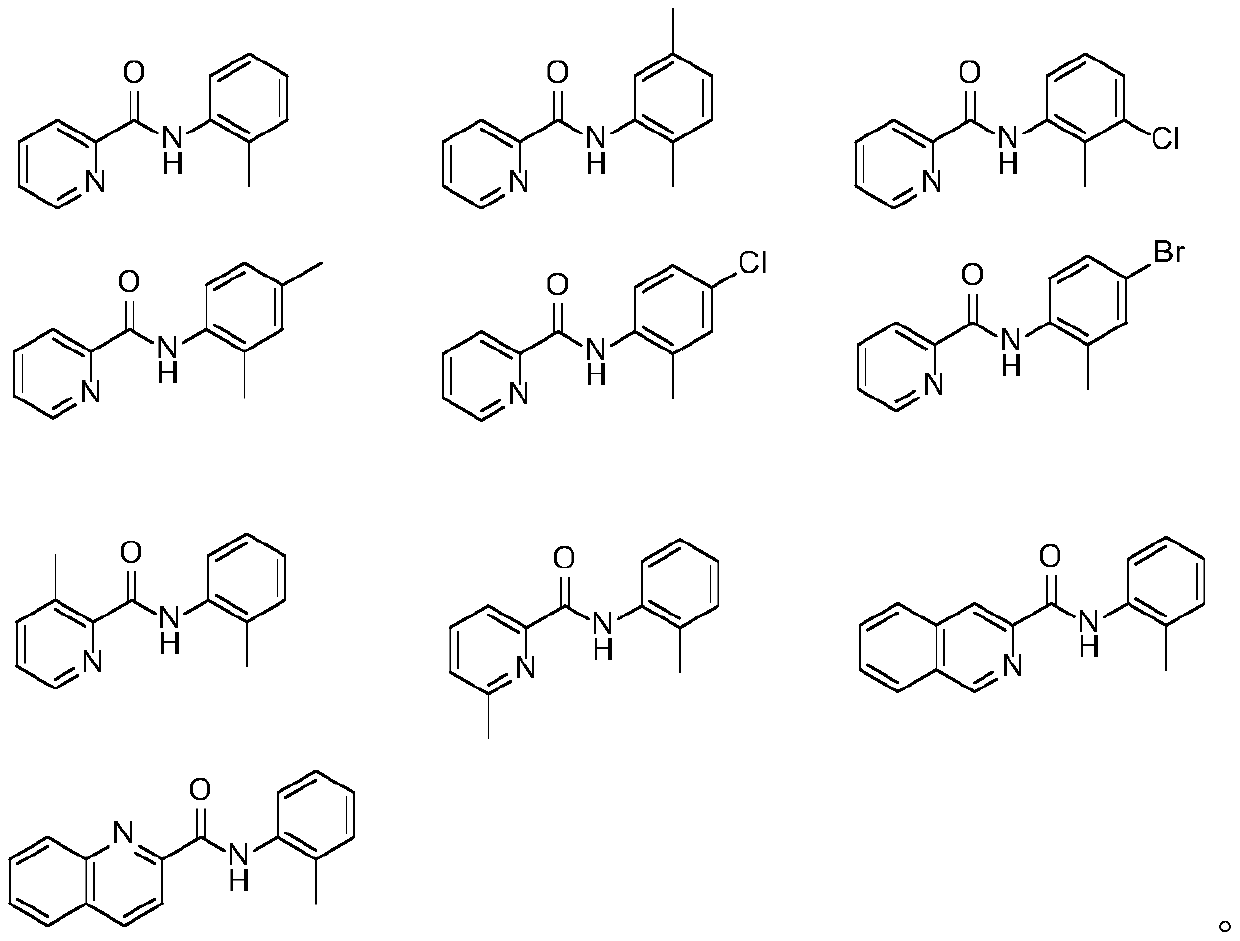
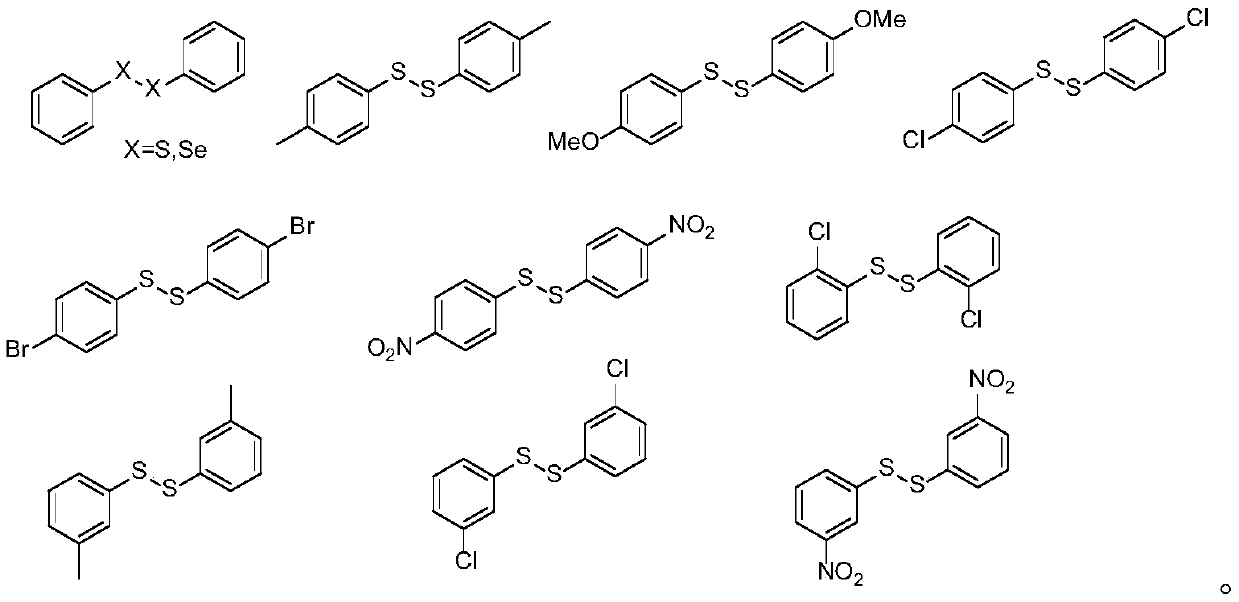
Synthesis method of o-toluenepicolinamide gamma-C-(sp<3>)H sulfur/selenide compound under catalysis of palladium
A technology of o-tolylamide and tolylamide, which is applied in the field of palladium-catalyzed synthesis of o-tolylamide γ-C-H sulfur/selenoether compounds, can solve the problems of poor atom economy and achieve high selectivity and product yield High and wide substrate applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030]
[0031] Add N-(o-tolyl) pyridine amide (4.24 grams, 20.0mmol), diphenyl disulfide (2.61 grams, 0.6eq), palladium acetate (449.0 milligrams, 10mol%), potassium acetate ( 3.92 g, 2.0 eq) and tert-butyl hydroperoxide (5.84 g, 2.0 eq), toluene (200.0 mL). Stir at 120 °C for 16.0 h under air atmosphere. Then the mixture was cooled to room temperature, filtered after cooling and concentrated under reduced pressure. Purified by silica gel column chromatography (developing solvent: petroleum ether: ethyl acetate = 40:1) to obtain 4.54 g of the target product as a colorless transparent oil, with a yield of 71%.
[0032] 1 H NMR (400MHz, CDCl 3 )δ10.68(s,1H),8.58(d,J=4.6Hz,1H),8.33(d,J=7.8Hz,1H),8.26(d,J=8.1Hz,1H),7.93(td, J=7.7,1.2Hz,1H),7.53–7.47(m,3H),7.38(t,J=7.4Hz,1H),7.32(t,J=7.1Hz,2H),7.26(d,J=7.2 Hz,1H),7.20 (d,J=7.3Hz,1H),7.09(t,J=7.5Hz,1H),4.24(s,2H). 13 C NMR (101MHz, CDCl 3 )δ162.36, 150.00, 148.13, 137.58, 136.12, 135.14, 131.80, 130.47, 128.90, 128.66, 12...
Embodiment 2
[0034]
[0035] Add N-(o-tolyl) pyridine amide (4.24 grams, 20.0mmol), p-toluene disulfide (2.95 grams, 0.6eq), palladium acetate (359.2 mg, 8mol%), potassium acetate ( 3.92 g, 2.0 eq) and tert-butyl hydroperoxide (5.84 g, 2.0 eq), p-xylene (100.0 mL). Stirred at 120°C for 12.0h under air atmosphere. Then the mixture was cooled to room temperature, filtered after cooling and concentrated under reduced pressure. Purified by silica gel column chromatography (developing solvent: petroleum ether: ethyl acetate = 40:1) to obtain 4.56 g of the target product as a colorless transparent oil, with a yield of 68%.
[0036] 1 H NMR (400MHz, CDCl 3)δ10.68(s,1H),8.65(d,J=4.4Hz,1H),8.35(d,J=7.8Hz,1H),8.27(d,J=8.1Hz,1H),7.95(t, J=7.2Hz, 1H), 7.53(dd, J=7.1, 4.8 Hz, 1H), 7.43(d, J=7.9Hz, 2H), 7.38(d, J=7.6Hz, 1H), 7.19(d, J=7.2Hz,1H), 7.15–7.09(m,3H),4.20(s,2H),2.36(s,3H). 13 C NMR (101MHz, CDCl 3 )δ162.35, 150.08, 148.13, 137.59, 137.54, 136.10, 132.57, 131.27, 130.48, 129.66, 128....
Embodiment 3
[0038]
[0039] Add N-(o-tolyl)pyridine amide (4.24 grams, 20.0mmol), 4,4'-dimethoxydiphenyl disulfide (3.90 grams, 0.7eq), palladium acetate (538.8 mg, 12mol%), potassium acetate (3.92g, 2.0eq) and tert-butyl hydroperoxide (5.84g, 2.0eq), toluene (80.0ml). Stirred at 110°C for 18.0h under air atmosphere. Then the mixture was cooled to room temperature, filtered after cooling and concentrated under reduced pressure. Purified by silica gel column chromatography (developing solvent: petroleum ether: ethyl acetate = 40:1) to obtain 5.4 g of the target product as a colorless transparent oil, with a yield of 77%.
[0040] 1 H NMR (400MHz, CDCl 3 )δ10.65(s,1H),8.69(d,J=4.3Hz,1H),8.36(d,J=7.8Hz,1H),8.27(d,J=8.0Hz,1H),7.99–7.93( m,1H),7.54(dd,J=6.9,5.2Hz,1H),7.46(d,J=8.6Hz,2H),7.41–7.35(m,1H),7.09(t,J=7.1Hz,2H ),6.84(d,J=8.6Hz,2H),4.14(s,2H),3.83(s,3H). 13 C NMR (101MHz, CDCl 3 )δ162.35, 159.72,150.11,148.13,137.56,136.01,135.34,130.53,128.85,128.46,127.29, 126.42,124.59,122...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com