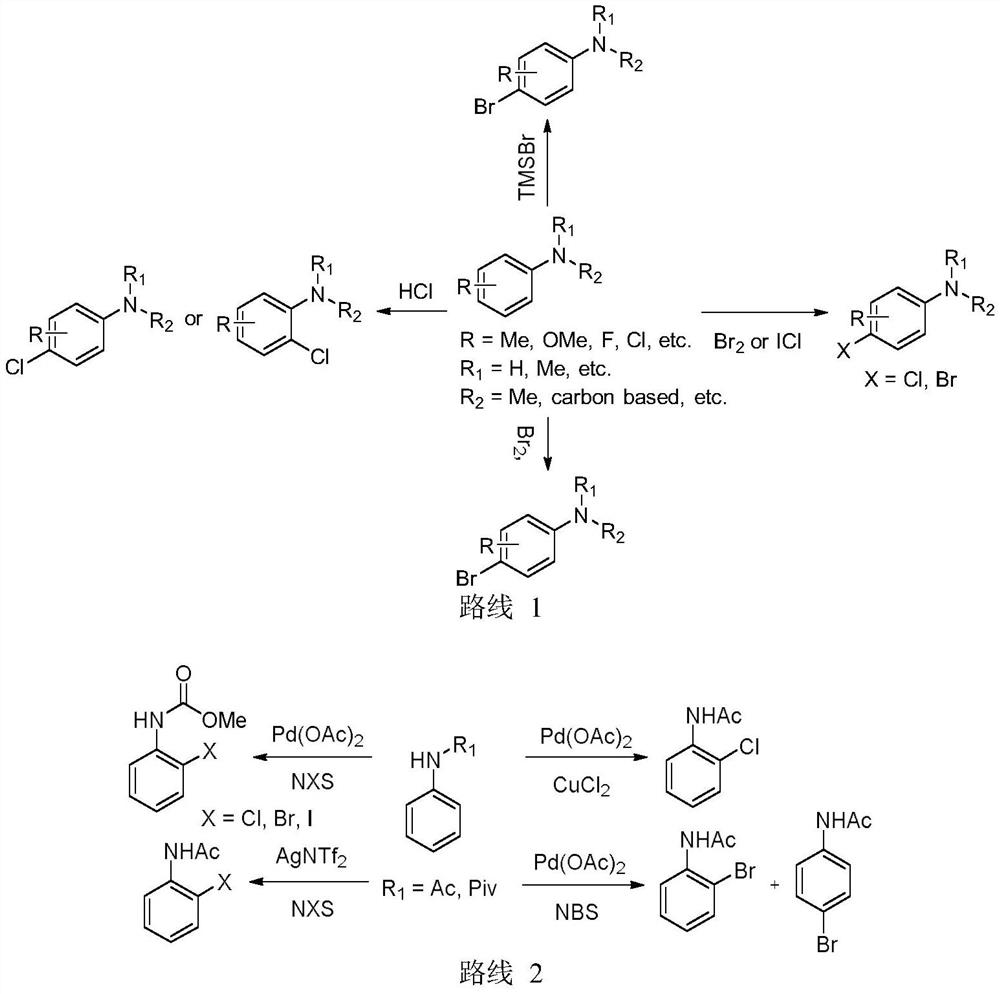
A kind of method for the selective synthesis of halogenated aromatic amines by copper catalysis
A halogenated arylamine and selective technology, which is applied in the field of copper-catalyzed selective synthesis of halogenated arylamines, to achieve the effects of high product yield, wide substrate applicability, and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
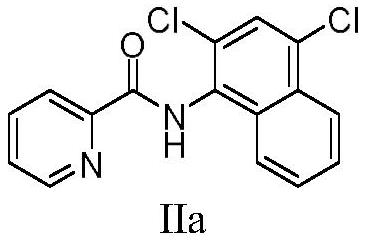
[0028] Add N-(naphthyl)pyridine amide (49.62mg, 0.2mmol), chlorosuccinimide (77.99mg, 0.6mmol), copper chloride (5.31mg, 20mol%), iodine acetate in a 25mL single-necked bottle Benzene (128.79 mg, 2.0 eq), N,N-dimethylformamide (4 mL). Stir at 60 °C for 3.0 h under air atmosphere. Then the mixture was cooled to room temperature, filtered after cooling and concentrated under reduced pressure. After purification by silica gel column chromatography (developing solvent: petroleum ether: ethyl acetate = 80:1), 39.19 mg of the target product was obtained as a white solid, with a yield of 62%.
[0029] 1 H NMR (400MHz, CDCl 3 )δ9.96(s,1H),8.71(d,J=4.4Hz,1H),8.33(d,J=7.6Hz,1H),8.28(d,J=8.7Hz,1H),7.95(t, J=7.8Hz, 2H), 7.70(s, 1H), 7.62(dd, J=13.8, 6.7Hz, 2H), 7.56(dd, J=7.3, 4.9Hz, 1H). 13 C NMR (101MHz, CDCl 3 )δ163.37,149.23,148.49,137.83,132.09,131.87,130.18,129.42,128.94,128.23,127.58,127.02,126.93,125.10,124.28,123.00.HRMS(ESI+):Calculated for 16 h 10 Cl 2 N 2 OH:[M+H] +...
Embodiment 2
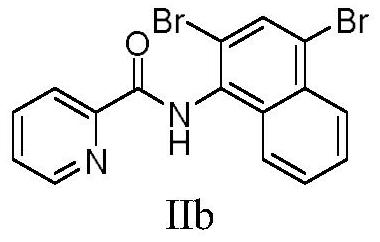
[0032] Add N-(naphthyl)pyridine amide (49.62mg, 0.2mmol), bromosuccinimide (83.49mg, 0.48mmol), copper bromide (8.83mg, 20mol%), iodine acetate in a 25mL single-necked bottle Benzene (128.79 g, 2.0 eq), N,N-dimethylformamide (4 mL). Stir at 60 °C for 3.0 h under air atmosphere. Then the mixture was cooled to room temperature, filtered after cooling and concentrated under reduced pressure. After purification by silica gel column chromatography (developing solvent: petroleum ether: ethyl acetate = 80:1), 61.40 mg of the target product was obtained as a white solid, with a yield of 76%.
[0033] 1 H NMR (400MHz, CDCl 3 )δ9.94(s,1H),8.71(d,J=4.7Hz,1H),8.33(d,J=7.8Hz,1H),8.24(d,J=8.4Hz,1H),8.05(d, J=1.6Hz, 1H), 7.95(dd, J=7.6, 5.8Hz, 2H), 7.63(t, J=7.6Hz, 1H), 7.60–7.52(m, 2H). 13 C NMR (101MHz, CDCl 3 )δ163.24, 149.21, 148.50, 137.82, 132.83, 132.10, 132.01, 131.78, 128.22, 128.00, 127.87, 127.04, 124.57, 122.99, 122.77, 119.51.HRMS(ESI+): Calculated for 16 h 10 Br 2 N 2...
Embodiment 3
[0036]Add 3-methyl-N-(naphthyl)pyridineamide (52.42mg, 0.2mmol), chlorosuccinimide (77.99mg, 0.6mmol), copper chloride (5.31mg, 20mol %), iodobenzene acetate (128.79 mg, 2.0 eq), N,N-dimethylformamide (4 ml). Stir at 60 °C for 3.0 h under air atmosphere. Then the mixture was cooled to room temperature, filtered after cooling and concentrated under reduced pressure. After purification by silica gel column chromatography (developing solvent: petroleum ether: ethyl acetate = 80:1), 49.50 mg of the target product was obtained as a white solid, with a yield of 75%.
[0037] 1 H NMR (400MHz, CDCl 3 )δ10.03(s,1H),8.23(d,J=8.3Hz,1H),8.13(d,J=7.0Hz,1H),8.05(s,1H),7.95(d,J=8.2Hz, 1H), 7.82(t, J=7.1Hz, 1H), 7.60(dt, J=14.8, 7.1Hz, 2H), 7.40(d, J=7.3Hz, 1H), 2.68(s, 3H). 13 C NMR (101MHz, CDCl 3 )δ164.70, 146.23, 145.79, 141.28, 136.59, 131.97, 131.68, 130.06, 129.83, 128.83, 128.12, 127.42, 126.84, 126.45, 124.97, 124.34, 20.82. 17 h 12 Cl 2 N 2 OH:[M+H] + 331.0399,Found: 331....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com