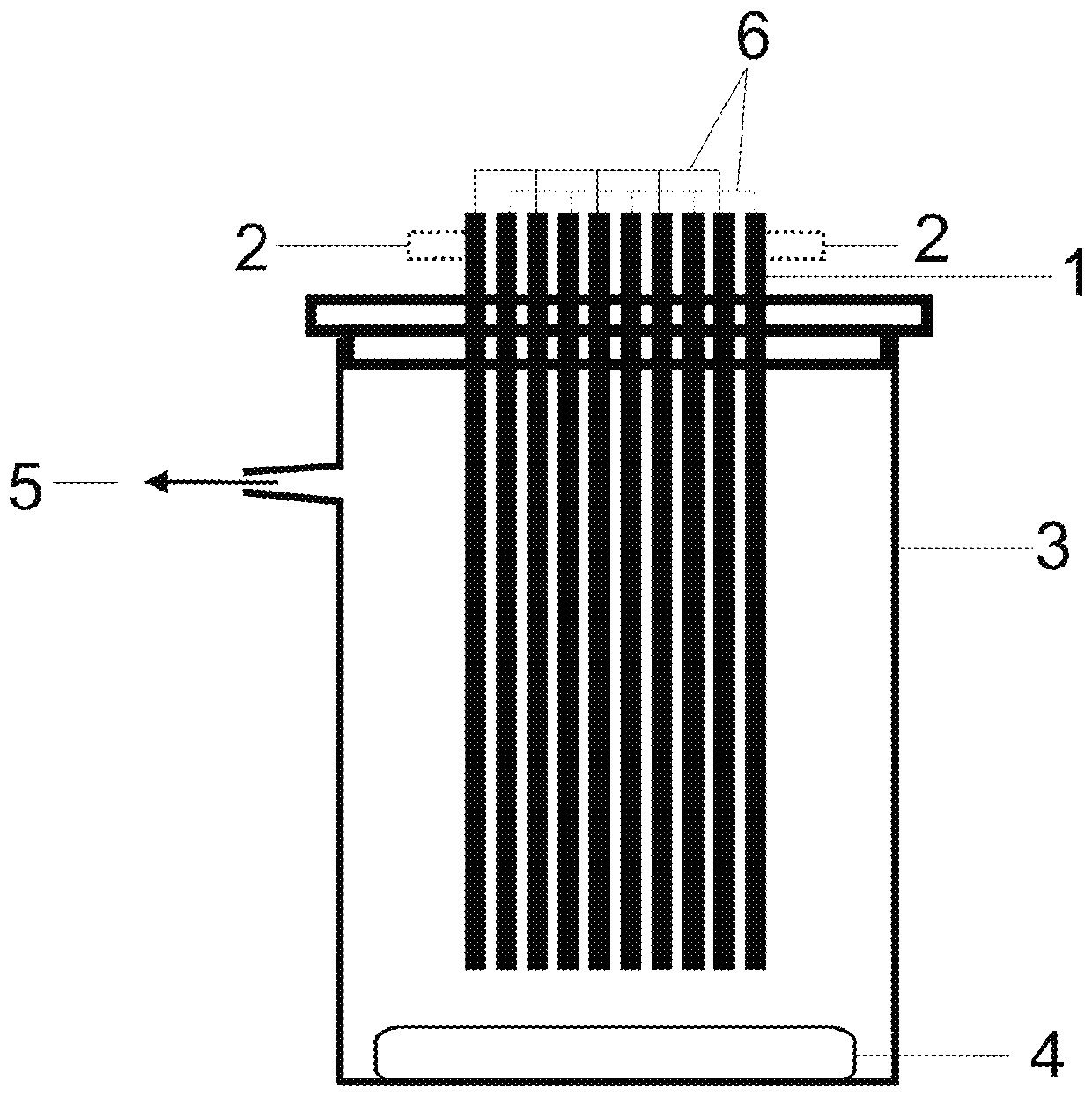
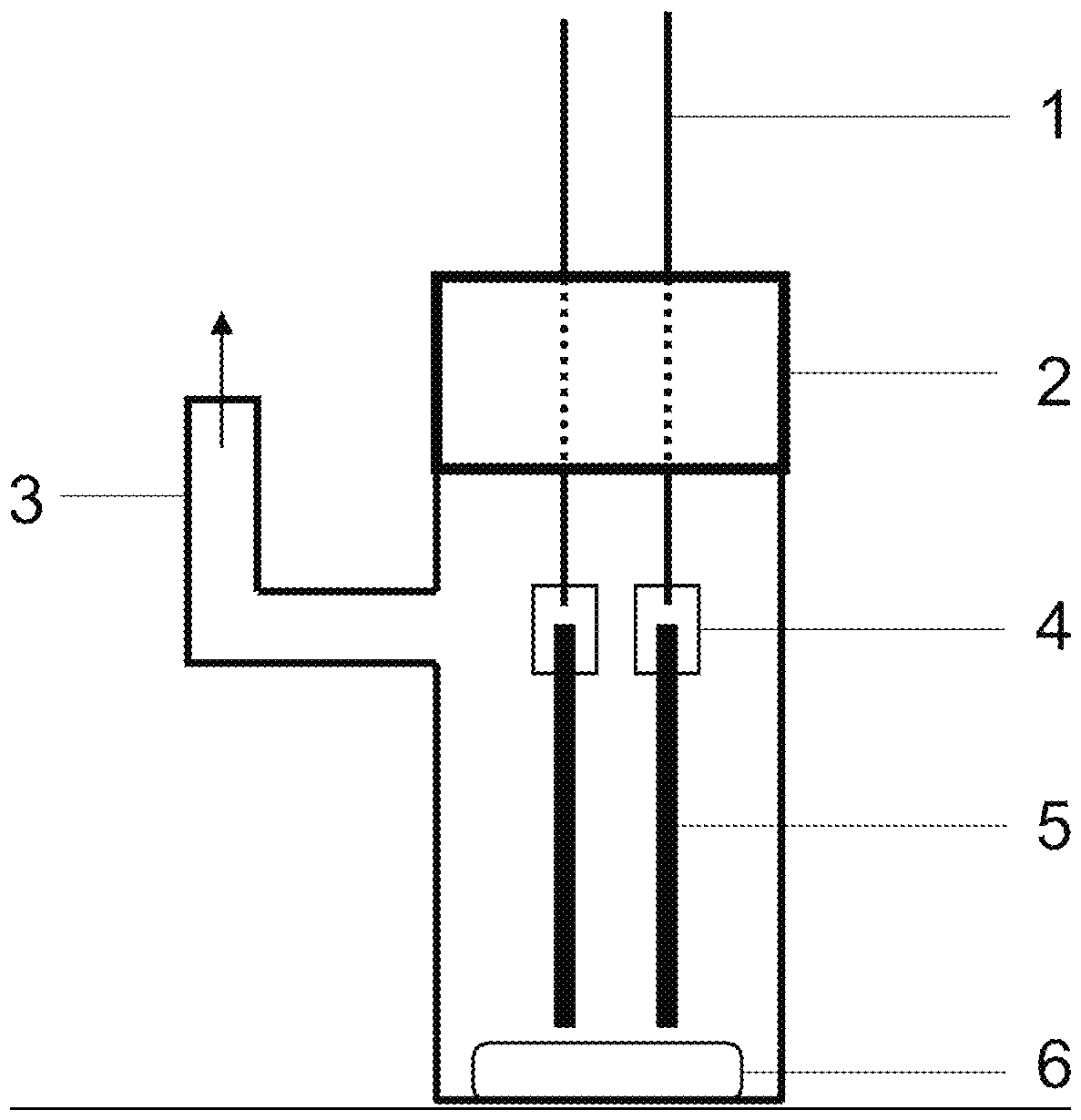
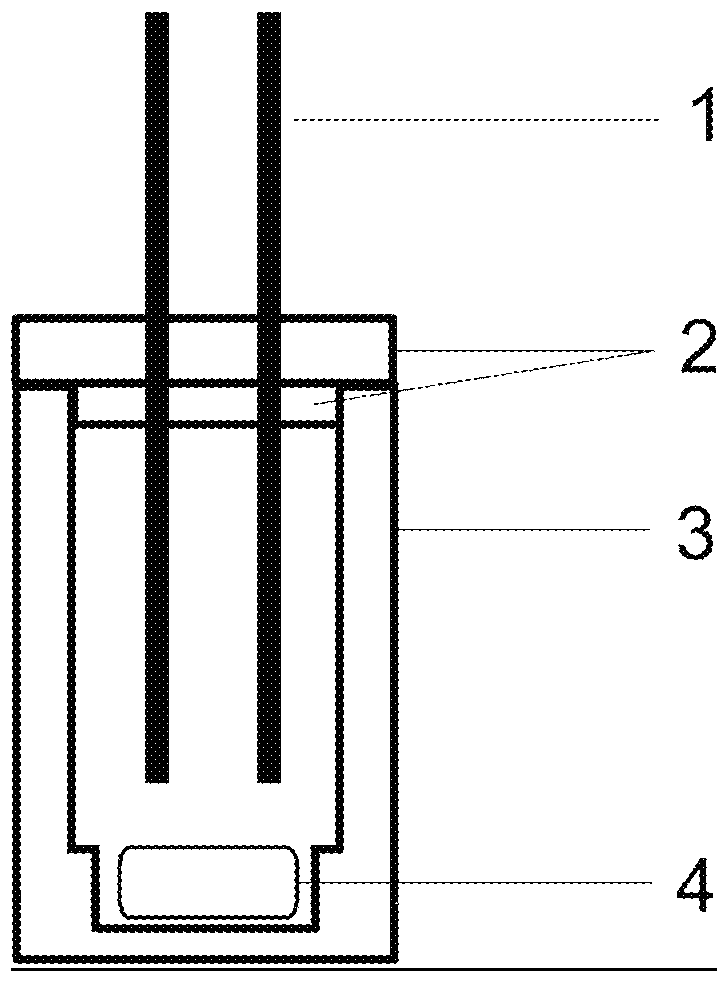
Process for preparing symmetric pincer ligands from group of m-terphenyl compounds
The technology of a compound and benzoyl group is applied in the field of compounds of the preparation formula ABA, which can solve the problems of high processing cost, disadvantage, and expensive ligand system, and achieves the effects of reducing the formation of by-products, direct synthesis is low, and consumption is reduced
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0119] Preparative liquid chromatography for the separation of mixtures of substances was performed using 60 M silica gel (0.040-0.063 mm) (Machery-Nagel GmbH & Co. KG, Düren) at a maximum pressure of 2 bar. All eluents used (ethyl acetate (technical purity), cyclohexane (technical purity)) were purified by distillation on a rotary evaporator beforehand.
[0120] Thin film chromatography (TLC) on ready-made PSC silica gel plates at 60 F 254 (Merck KGaA, Darmstadt). Detection of the different substances first under UV light and then by means of staining with the cerium / molybdophosphoric acid reagent (5.6 g of molybdophosphoric acid, 2.2 g of cerium(IV) sulfate tetrahydrate and 13.3 g of concentrated sulfuric acid in 200 ml of water) and then Carry out by heating with a hot hair dryer.
[0121] Gas Chromatography (GC / GCMS)
[0122] Gas chromatography (GC) analysis of product mixtures and pure substances was performed with the aid of a gas chromatograph GC-2010 (Shimadzu, Ja...
Embodiment 1
[0144] 2,2''-dihydroxy-5,5''-dimethyl-2'- N,N -Dimethylamino-3,3'',4'-trimethoxy[1,1';5'1''] Terphenyl (1)
[0145] The electrolysis was carried out according to AAV1, using 1.38 g (10 mmol, 2.0 equiv) of 4-methylguaiacol and 0.76 g (5 mmol, 1.0 equiv) of 3-( N , N -Dimethylamino)anisole. The current intensity is 15 mA, and the charge is 1929.7 C (4F per 3-( N , N -Dimethylamino)anisole). The crude product was prepurified by column chromatography on silica gel 60 using a gradient of eluent mixture of 95:5, then 9:1 and finally 4:1 (cyclohexane (CH):ethyl acetate (EE)). The fractions obtained were tested for the presence of product and re-used on silica gel 60 with eluents 1:1:0.01, then 1:1:0.02 (dichloromethane (DCM):cyclohexane (CH):methanol ( MeOH)) purification. Additional purification of the mixed fractions was by bulb distillation (150 °C, 180 °C, 190 °C and 205 °C for 10 -3 mbar) to proceed. The product was obtained as a yellow oil.
[0146]
[0147]...
Embodiment 2
[0155] 2,2''-dihydroxy-3,3'',4'-trimethoxy-5,5'',2'-trimethyl[1,1';5'1'']terphenyl (2 )
[0156] The electrolysis was carried out according to AAV1, using 1.45 g (10.5 mmol, 2.0 equiv) of 4-methylguaiacol and 639 mg (5.23 mmol, 1.0 equiv) of 3-methylanisole. Current density of 2.8 mA / cm 2 , with a charge of 2 F per 4-methylguaiacol (Q = 2018 C). After separation of the solvent, the product mixture was purified by column chromatography on silica gel 60 using eluent 9:1 (CH:EE). The product was obtained as a yellow oily substance.
[0157]
[0158] Yield: 68 mg (0.17 mmol, 3%)
[0159] GC (hard method, HP-5): t R = 21.35 min
[0160] R f (Cy:EA = 4:1) = 0.39
[0161]
[0162]
[0163] HRMS (ESI, positive mode): m / z C 24 h 26 o 5 (M+Na + ): Calculated: 417.1678; Measured: 417.1670
[0164] Melting point: 67.7°C (crystallization from DCM).
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| strength | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com