Synthetic method of aryl sulfhydryl diazole derivative
A technology of arylmercaptodiazoles and mercaptodiazoles, which is applied in the field of organic synthesis, can solve problems such as unsuitable for popularization and application, cumbersome preparation process, harsh conditions, etc., and achieve ideal conversion rate and selectivity, safe operation, and reaction The effect of mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
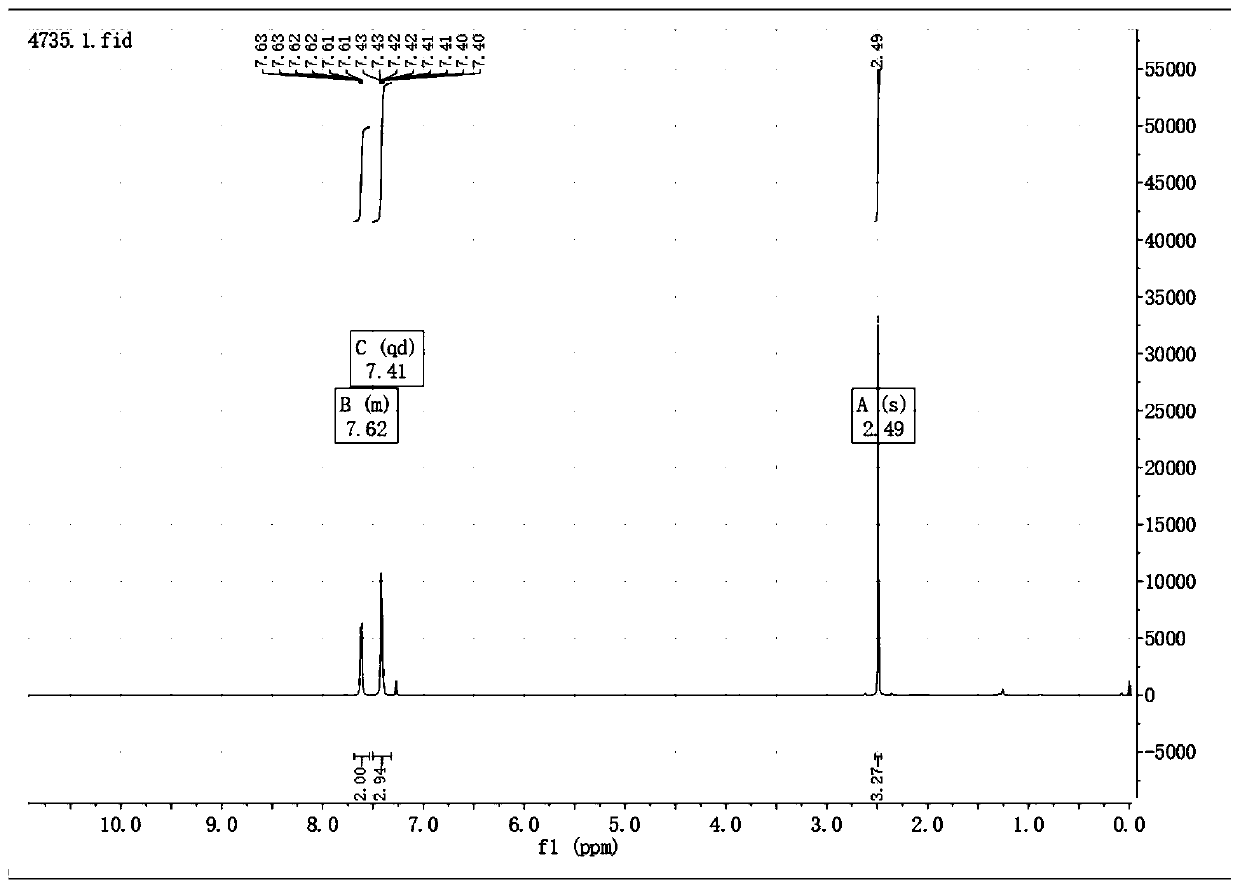
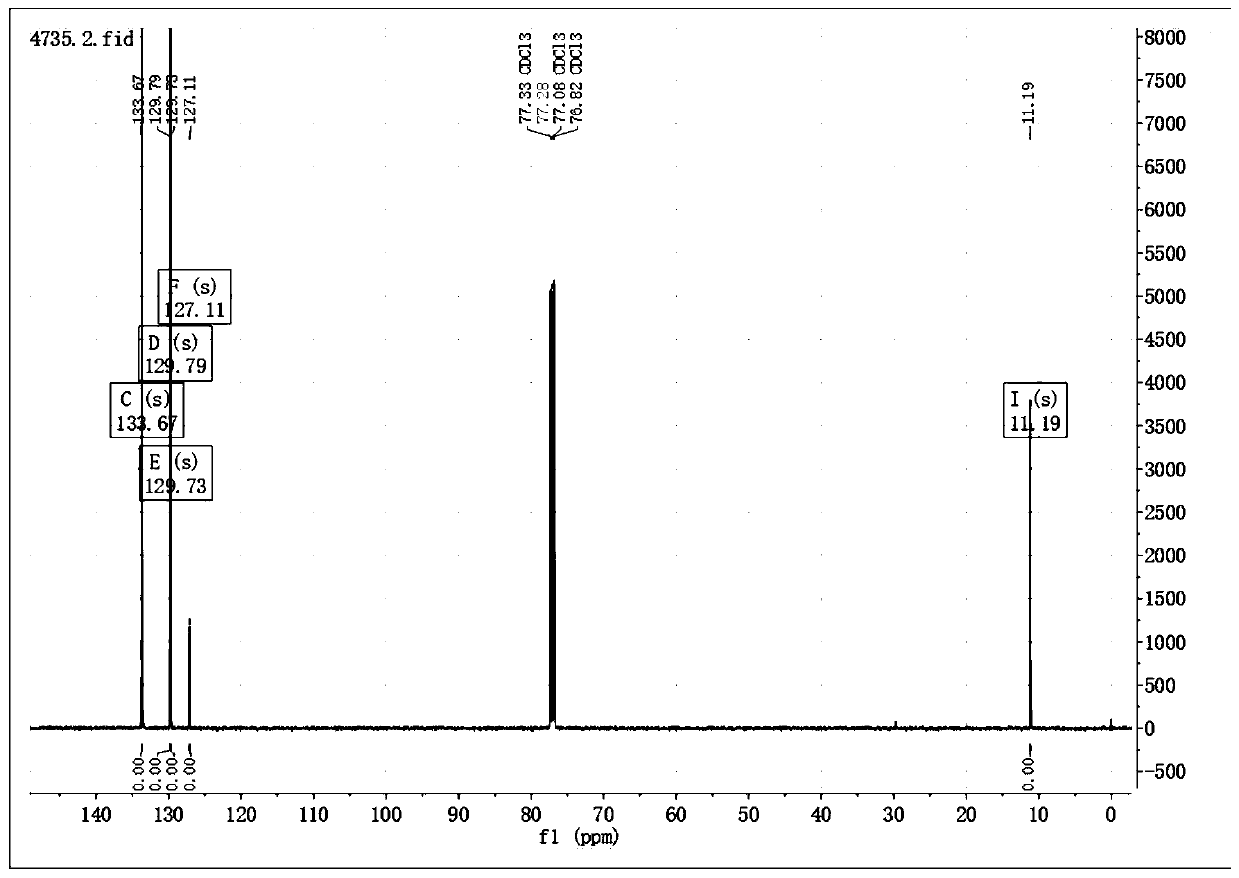
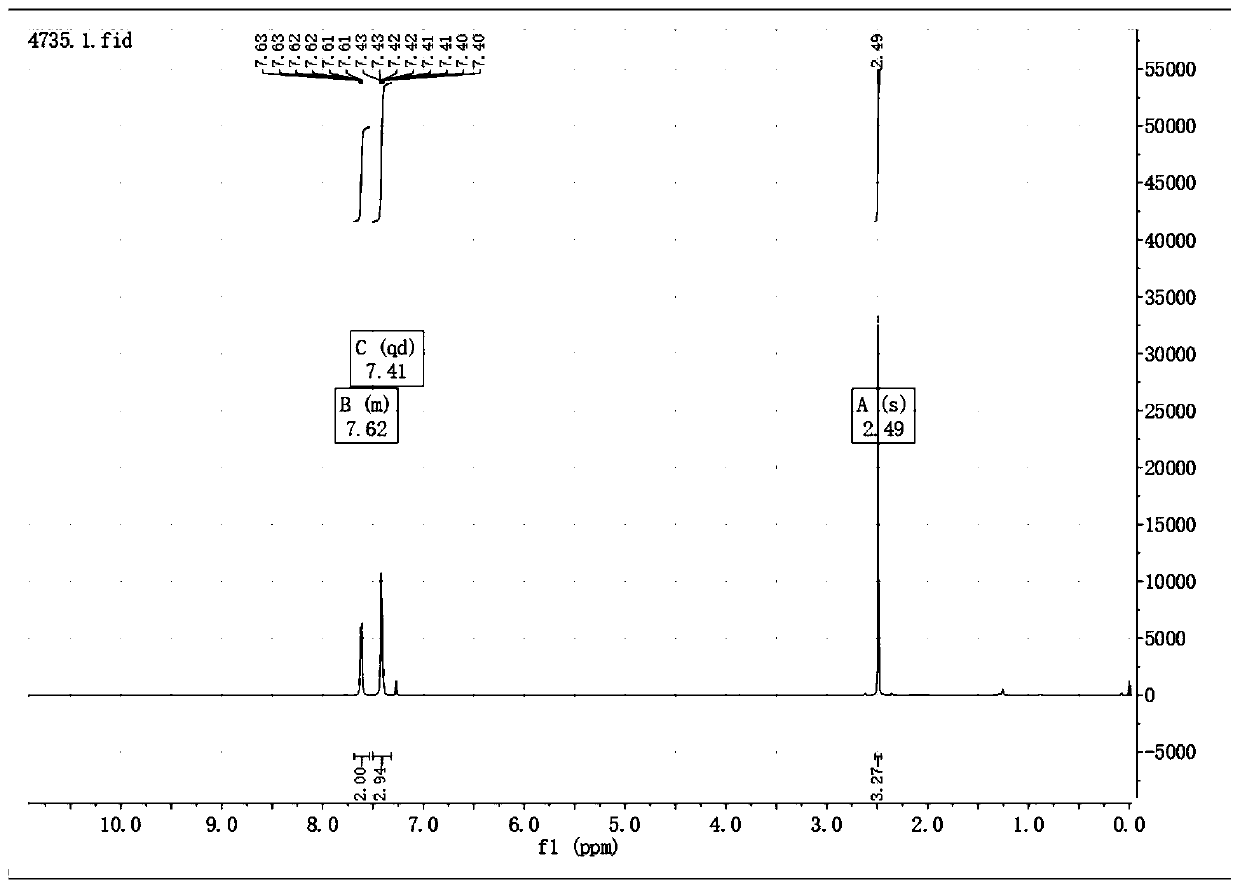
[0034] When connecting the thermometer, rubber gland and condenser tube, N 2 Add 0.033g (0.28mmol) 5-methyl-2-mercapto-1,3,4-oxadiazole, 0.1g (2.4eq) cesium fluoride and 3ml acetonitrile into the three-necked flask of the balloon, seal the device and use N 2 Replace three times, start stirring, and heat up to reflux. Inject 0.1 g (1.2 eq) of benzyne precursor 2-trimethylsilyl phenyl trifluoromethanesulfonate with a syringe through the rubber stopper, keep the reflux reaction for 6 h, stop heating and stirring, and cool to room temperature. Add 20ml of water, extract 3 times with 10ml ethyl acetate, combine the organic layers, wash 2 times with 10ml saturated NaCl solution, 1g anhydrous MgSO 4 Dry, filter, concentrate to remove the solvent, use EA:PE=1:2 column chromatography to obtain the pure product 5-methyl-2-phenylmercapto-1,3,4-oxadiazole 0.05g, yield 92.9%, It is a yellow solid.
Embodiment 2
[0036] When connecting the thermometer, rubber gland and condenser tube, N 2Add 0.037g (0.28mmol) 5-methyl-2-mercapto-1,3,4-thiadiazole, 0.1g (2.4eq) cesium fluoride and 3ml acetonitrile into the three-necked flask of the balloon, seal the device and use N 2 Replace three times, start stirring, and heat up to reflux. Inject 0.1 g (1.2 eq) of benzyne precursor 2-trimethylsilyl phenyl trifluoromethanesulfonate with a syringe through the rubber stopper, keep the reflux reaction for 6 h, stop heating and stirring, and cool to room temperature. Add 20ml of water, extract 3 times with 10ml ethyl acetate, combine the organic layers, wash 2 times with 10ml saturated NaCl solution, 1g anhydrous MgSO 4 Dry, filter, concentrate to remove solvent, use EA:PE=1:5 column chromatography to obtain 0.058g of pure product 5-methyl-2-phenylmercapto-1,3,4-thiadiazole, the yield is close to quantitative reaction , as a white solid.
Embodiment 3
[0038] When connecting the thermometer, rubber gland and condenser tube, N 2 Add 0.05g (0.28mmol) 5-phenyl-2-mercapto-1,3,4-oxadiazole, 0.1g (2.4eq) cesium fluoride and 3ml acetonitrile into the three-necked flask of the balloon, seal the device and use N 2 Replace three times, start stirring, and heat up to reflux. Inject 0.1 g (1.2 eq) of benzyne precursor 2-trimethylsilyl phenyl trifluoromethanesulfonate with a syringe through the rubber stopper, keep the reflux reaction for 6 h, stop heating and stirring, and cool to room temperature. Add 20ml of water, extract 3 times with 10ml ethyl acetate, combine the organic layers, wash 2 times with 10ml saturated NaCl solution, 1g anhydrous MgSO 4 Dry, filter, concentrate to remove the solvent, use EA:PE=1:5 column chromatography to obtain the pure product 5-phenyl-2-phenylmercapto-1,3,4-oxadiazole 0.06g, yield 84.3%, It is a yellow solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com