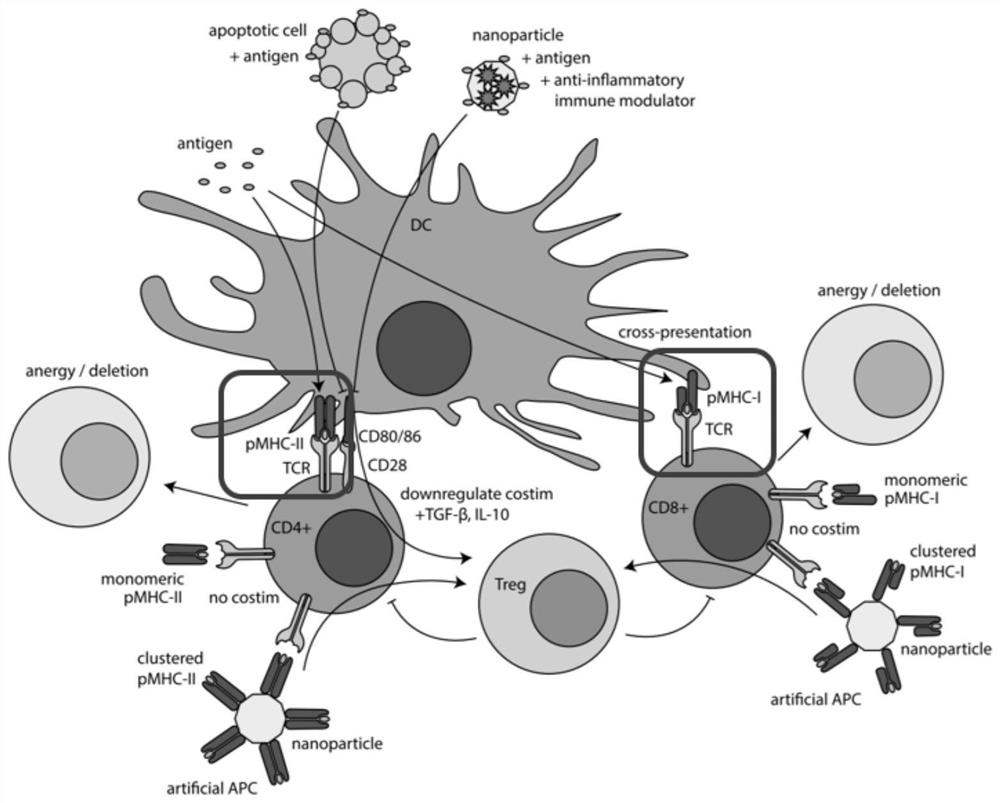
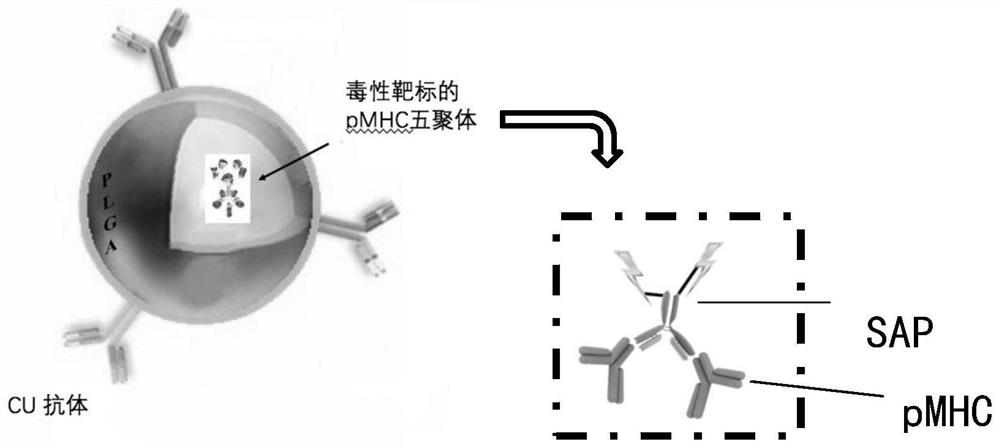
Dual-targeted nanomedicine for customized T-cell epitope vaccine, preparation method and application thereof
An epitope vaccine and dual-targeting technology, applied in nanotechnology, nanomedicine, and nanotechnology for materials and surface science, can solve problems such as uncontrollable drug release, reduce degradation and loss, and reduce toxic and side effects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] (1) Preparation of targeted killer polymer
[0052] 1) Customized specific targeting polymer: select the pathogenic CD8+ T cell-specific antigen peptide IGRP206-214 of Tcra TcrbNY8.3 mouse, and synthesize the corresponding biotinylated MHC Class I polymer with mouse H-2kd Body (Proimmune, UK).
[0053] 2) Select the streptomycin / saporin complex (Streptavidin-ZAP), mix it with the customized biotin-labeled pMHC pentamer in an equimolar ratio, mix well and incubate at room temperature for 15 minutes, then place the mixture in- Store at 20°C, shake gently before use, and dilute as needed.
[0054] (2) Preparation of targeted nano-drugs
[0055] 1) Dissolve PLGA (300 mg) in DCM (20 mL), emulsify the targeted killer polymer solution into the PLGA solution using a probe sonicator, and set the amplitude at 40% for 100 s in an ice bath.
[0056] 2) Pour the W / O colostrum into the PVA solution (3%), and homogenize it in an ice bath at 6000 rpm for 30 minutes using a high-spee...
Embodiment 2
[0064] (1) Preparation of targeted killer polymer
[0065] 1) Customized specific targeting polymers: clinically, different patients screen out the pathogenic CD8+ T cells with dominant expression in their body according to their peripheral blood, and customize the individual according to the epitope polypeptide type of the pathogenic T cells MHC Class I polymer (pMHC multimer) (Proimmune, UK) marked with the polypeptide-Tag sequence.
[0066] 2) Mix the pMHC multimer linked with the Tag sequence and the saponin protein linked with the anti-Tag sequence in an equimolar ratio, mix thoroughly and incubate at room temperature for 15 minutes, then store the mixture at -20°C, and gently wash it before use. Shake to dilute as needed.
[0067] (2) Preparation of targeted nano-drugs
[0068] 1) DPPC, DSPC, and DSPE-PEG2K were fully dissolved in chloroform at a molar ratio of 90:5:5, then protein G antibody (Abcam, UK) was added, and mixed well.
[0069] 2) Evaporate in vacuo under ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com