Process for preparing adipic dialdehyde from cyclohexene
A technology for cyclohexene and adipaldehyde, which is applied in the field of preparing adipaldehyde from cyclohexene, can solve the problems of high price, excessive oxidation, hindering industrial application, etc., and achieves high product selectivity, efficient mass transfer, and enhanced material transfer. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Cyclohexene is oxidized to adipaldehyde in the microchannel reactor of embodiment 1
[0054] Mix 50g of cyclohexene and 116g of acetone to prepare a cyclohexene solution with a mass concentration of 30%, add 7.78g of ruthenium chloride trihydrate and 6.08g of methyl pyruvate into the cyclohexene solution, and mix well by ultrasonic vibration After that, the mixed solution is obtained;
[0055] A pump is used to inject the mixed solution into the microchannel reactor, the mixed solution flow rate is 10g / min, the mixed gas is injected into the microchannel reactor through a mass flow meter, and the controlled mixed gas flow rate is 15.1L / min, wherein the mixed gas is composed of oxygen and Ozone composition, the ozone concentration is 100mg / L;
[0056] The reaction temperature in the microchannel reactor is 10° C., and the reaction pressure is 0.10 MPa. Samples were taken after 10 minutes of reaction after feeding, and analyzed by gas chromatography.
[0057] Cyclohexe...
Embodiment 14-17
[0060] It is basically the same as Example 1-4, the only difference is that no auxiliary agent is used, and the specific reaction conditions and results are shown in Table 1.
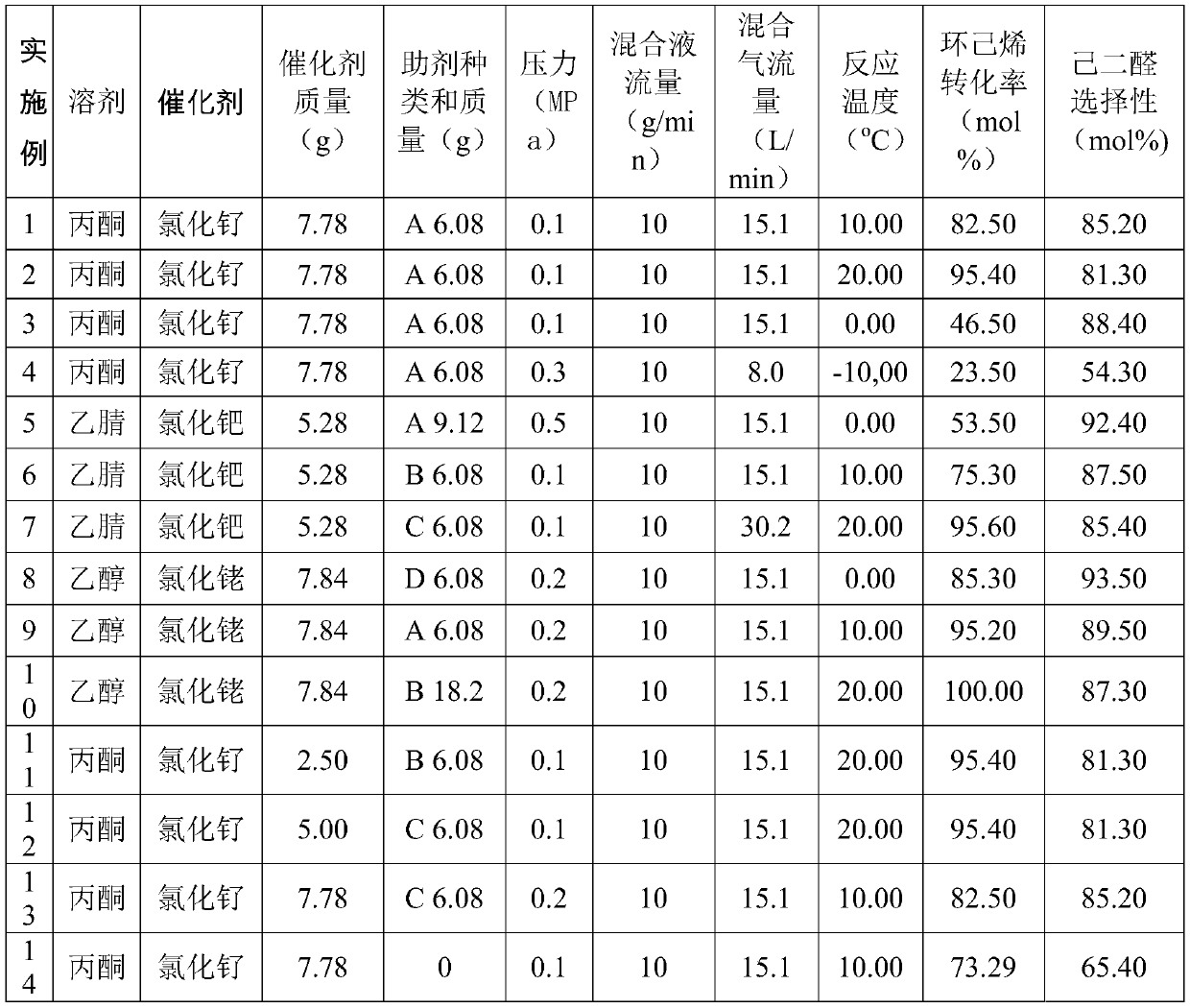
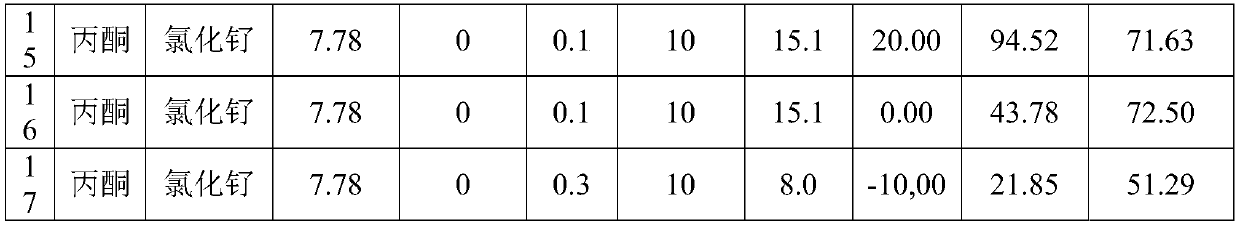
[0061] Table 1 Reaction conditions and result list of cyclohexene oxidation to adipaldehyde in microchannel reactor
[0062]
[0063]
[0064] Remarks: Auxiliary A: methyl pyruvate, Auxiliary B: methyl trifluoropyruvate, Auxiliary C: methyl acetylacetonate, Auxiliary D: methyl acetoacetate.
[0065] It can be seen from Table 1 that in the preparation method provided by the application, adipaldehyde generally has a higher selectivity, especially when using auxiliary agents and the reaction temperature is 0-20°C, the selectivity of adipaldehyde can reach more than 81.3%, the highest It can reach 93.5%, and the conversion rate of cyclohexene can reach more than 85.3%, and the highest can reach 100%. The selectivity of adipaldehyde in the embodiment of the auxiliary agent is higher than that in the em...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com