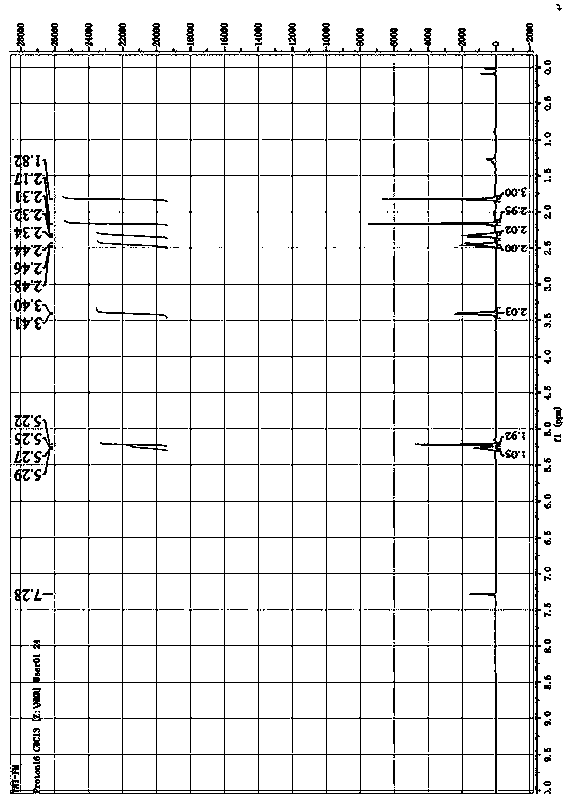
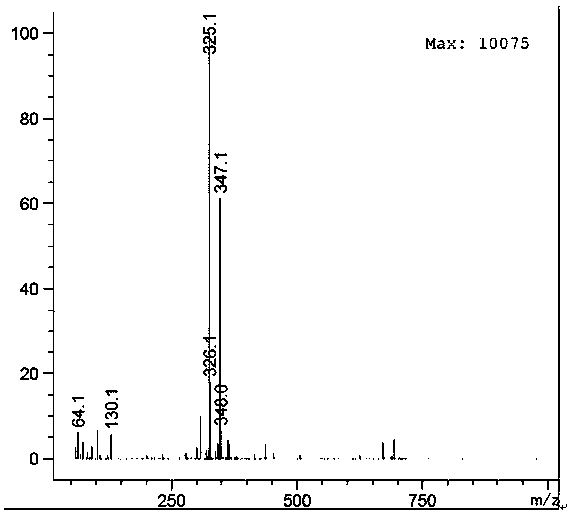
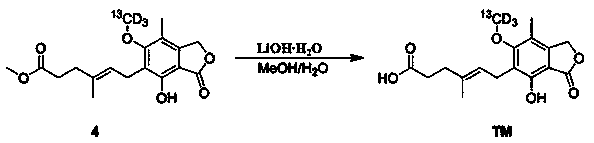Synthesis method of mycophenolic acid-13CD3
A synthesis method, mycophenolic acid technology, applied in the fields of organic chemistry, organic chemistry, bulk chemical production, etc., can solve the problems of long reaction route, high cost, long route, etc., and achieve the effect of controllable cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Synthesis of mycophenolic acid- 13 CD3
[0032] step 1)
[0033] The synthetic route is as follows:
[0034]
[0035] N 2 Under protection, 7.5g (23.4mmol) of compound 1, 15mL pyridine (186.4mmol), 74mL 2,4,6-collidine (560mmol), and 21.2g (158.4mmol) of lithium iodide were sequentially added to a 250 mL single-necked bottle 48 hours at 135°C, followed by plate monitoring. After the reaction of the raw materials was complete, cool the system and adjust the pH to about 4 with dilute hydrochloric acid, then extract with ethyl acetate, combine the organic phases, and wash the organic phases once with 0.1M dilute hydrochloric acid. Washed once with saturated brine, dried over anhydrous sodium sulfate, and spin-dried the solvent to obtain 7.5 g of a light yellow solid with a yield of 95.6%. The reaction product was directly used in the next reaction.
[0036] step 2)
[0037] The synthetic route is as follows:
[0038]
[0039] Dissolve 7.0g (22.8mmol) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com