Marine fungus, novel skeleton meroterpenoid derivatives prepared from marine fungus as well as preparation method and application of novel skeleton meroterpenoid derivatives
A technology of marine fungi and derivatives, applied in the field of pharmaceutical compounds, can solve problems such as liver and kidney damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The acquisition of embodiment 1 marine fungus Aspergillus terreus GZU-31-1
[0041] 1. Isolation of strains: The inventor's team isolated and purified a marine fungus, Aspergillus terreus GZU-31-1, from the trunk of sulphurous sulphur, along the coast of Xuwen, Zhanjiang City, Guangdong Province.
[0042] The specific separation process is as follows: wash the back stone with clear water, sterilize the surface with 1% sodium hypochlorite aqueous solution for 30 seconds, and take it out. After sterilizing with 75% alcohol for 30 seconds, rinse with water. The slices were placed on Bengal red medium for culture and growth until a single colony grew.
[0043] 2. Identification of strains
[0044] Aspergillus terreus GZU-31-1 strain showed yellow filamentous fungal colonies on PDA medium.
[0045] Molecular identification:
[0046]The fungus was identified by DNA amplification and sequencing of the ITS region of the fungus. For specific steps, refer to the literature (N...
Embodiment 2
[0049] The separation of embodiment 2 compound
[0050] A novel skeleton heteroterpene derivative was isolated from the fermentation broth of the marine fungus Aspergillus terreus GZU-31-1. The specific method is as follows:
[0051] (1) Seed liquid culture of the fungus Aspergillus terreus GZU-31-1: The composition of the medium is by weight: 0.3% glucose, 0.1% yeast extract, 0.5% peptone, 2.5% agar, 3% sodium chloride, and 98% water %; Make a test tube slant, pick the strains and insert them into the slant, and cultivate them at 30°C for 6 days;
[0052] (2) Fermentation culture of the fungus Aspergillus terreus GZU-31-1: using solid rice fermentation medium: rice: seawater = 1:1 (mass ratio); ℃ for 2 months;
[0053] (3) extracting the above-mentioned fermented thallus with methanol for 3 times, concentrating the extract, and extracting the obtained concentrated extract with ethyl acetate to obtain a crude ethyl acetate extract;
[0054] (4) The ethyl acetate crude extra...
Embodiment 3
[0056] The structural analysis of embodiment 3 compound
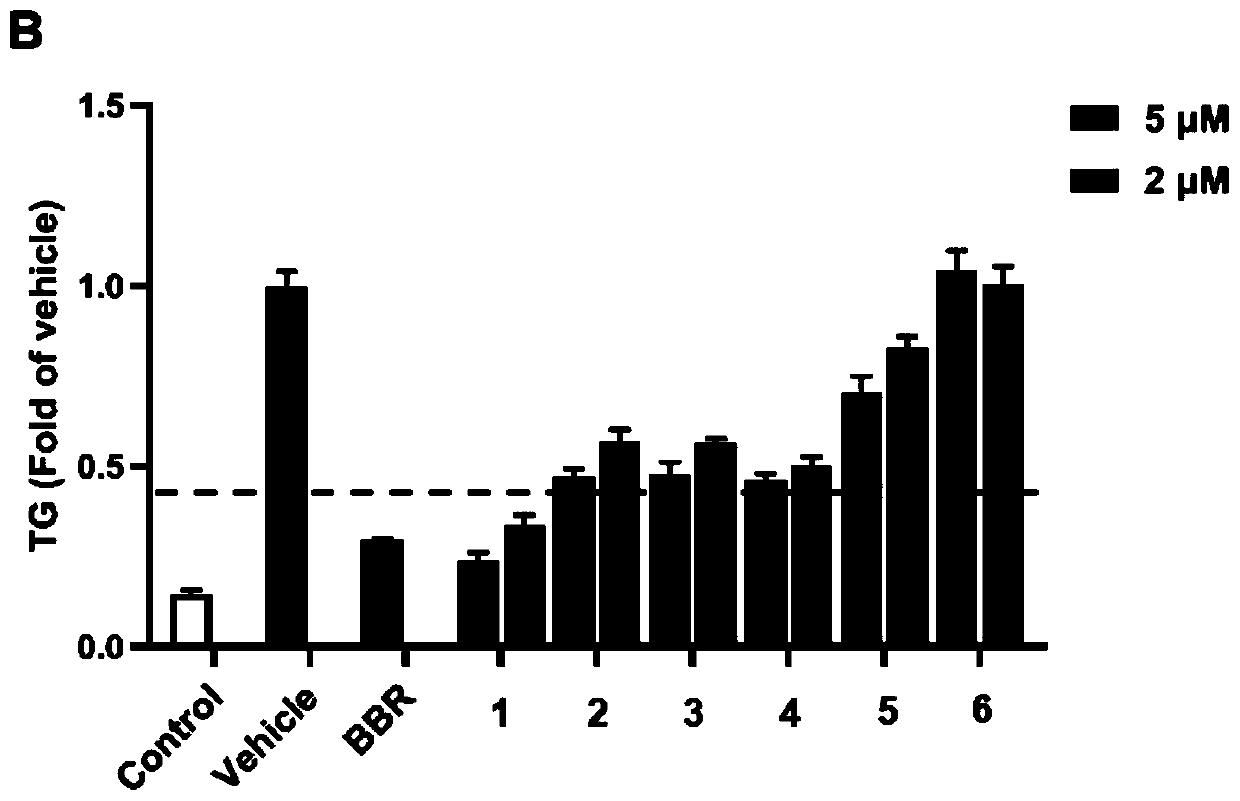
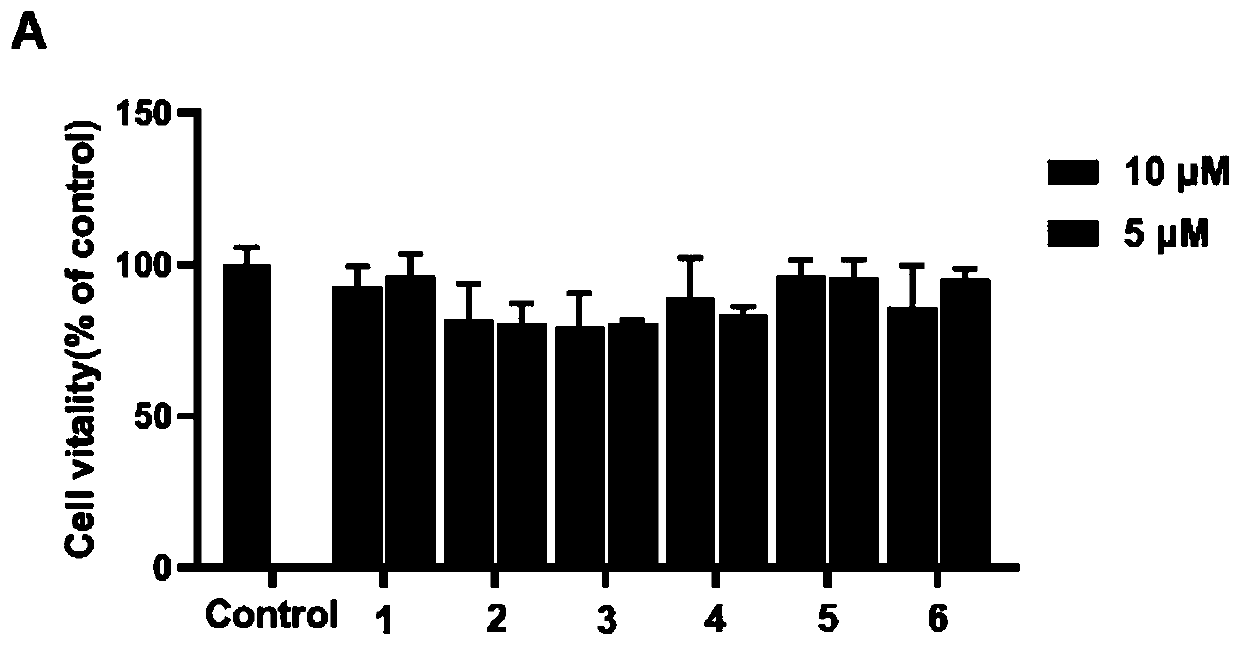
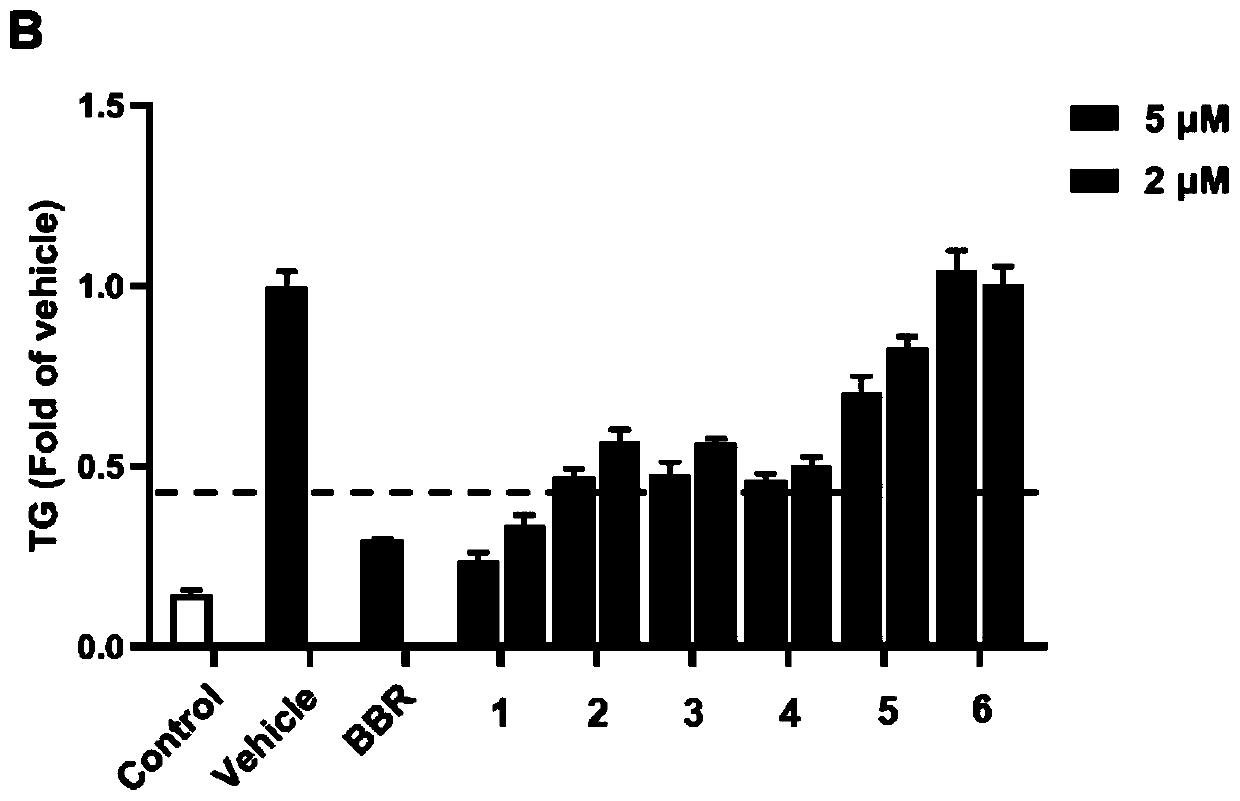
[0057] Structural analysis of new compounds 1-6 was carried out, and the following experimental data were obtained:
[0058] New Compound 1: C 28 h 32 o 10 , HRESI-MS: 527.1912 [M-H] - (cald for C 28 h 31 o 10 527.1917).
[0059] New compound 2: C 28 h 32 o 10 , HRESI-MS: 527.1913 [M-H] - (cald for C 28 h 31 o 10 527.1917).
[0060] New Compound 3: C 28 h 32 o 10 , HRESI-MS: 527.1914 [M-H] - (cald for C 28 h 31 o 10 527.1917).
[0061] New Compound 4: C 28 h 34 o 10 , HRESI-MS: 529.2056 [M-H] - (cald for C 28 h 33 o 10 529.2073).
[0062] New Compound 5: C 28 h 34 o 11 , HRESI-MS: 545.2022 [M-H] - (cald for C 28 h 33 o 11 545.2022).
[0063] New compound 6: C 28 h 32 o 11 , HRESI-MS: 543.1932 [M-H] - (cald for C 28 h 31 o 11 543.1932).
[0064] The structural formulas and NMR data of compounds 1-6 are as follows:
[0065]
[0066] Table 1. NMR data of compounds 1-3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com