A kind of preparation method and application of fluoroolefin
A fluoroolefin and reaction tube technology, which is applied in the field of preparation of fluoroolefins, can solve the problems of insufficient selectivity, complex catalytic system, low reaction activity, etc., and achieves high selectivity, simple catalytic system and wide reaction range. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
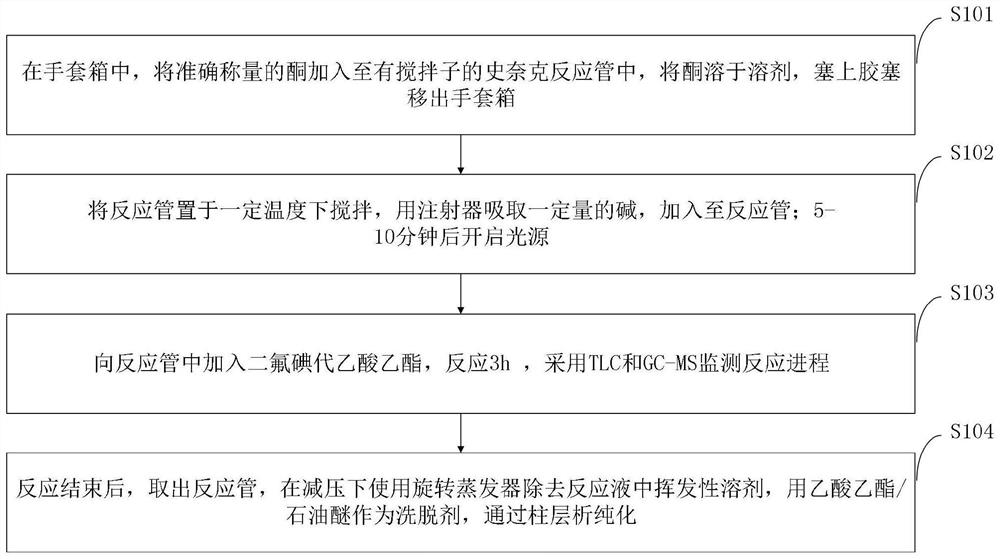
[0043] The preparation method of the fluoroolefin provided by the embodiment of the present invention comprises:
[0044] Under the catalysis of light, the difluoroalkylation-defluorination reaction using ethyl difluoroiodoacetate as the fluorine source, and the high stereoselective synthesis of fluoroalkenes by ketonization of aryl benzyl.
[0045] Such as figure 1 As shown, the preparation method of the fluoroolefin provided by the embodiment of the present invention comprises the following steps:
[0046] S101, in the glove box, add the accurately weighed ketone into the Schneck reaction tube with a stirring bar, dissolve the ketone in the solvent, put on a rubber stopper and take it out of the glove box.
[0047] S102. Stir the reaction tube at a certain temperature, draw a certain amount of alkali with a syringe, and add it to the reaction tube; turn on the light source after 5-10 minutes.
[0048] S103, adding ethyl difluoroiodoacetate into the reaction tube, reacting...
Embodiment 1
[0057]
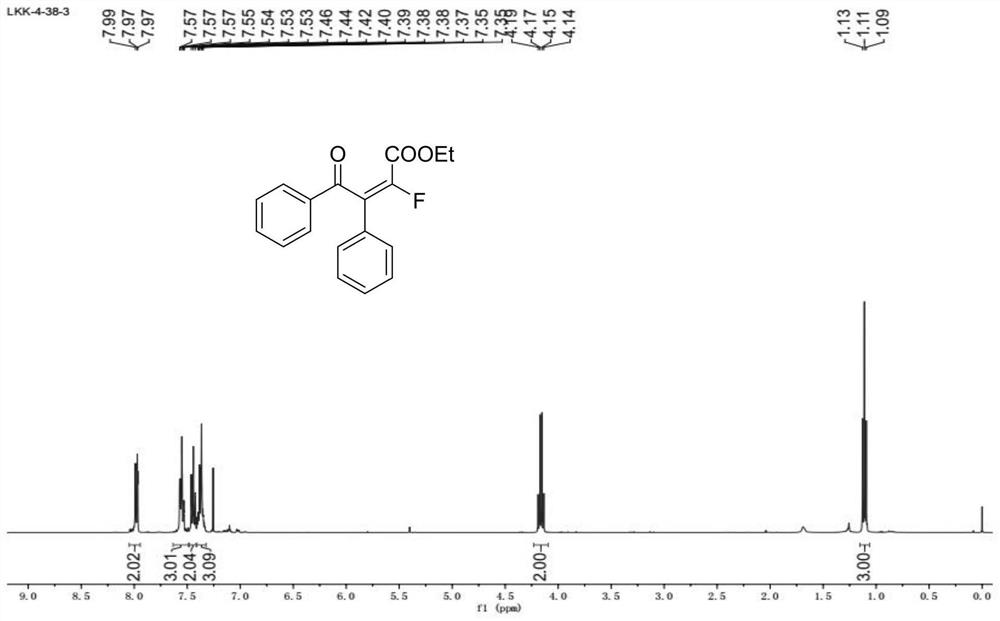
[0058] In the glove box, accurately weigh 2-phenylacetophenone (0.2 mmol) and add it to a Schneck reaction tube with a stirring bar, dissolve it in tetrahydrofuran, put on a rubber stopper and remove it from the glove box. The reaction tube was stirred at -20° C., and then sodium bis(trimethylsilyl)amide (0.32 mmol) was added to the reaction tube with a syringe. Turn on the LED blue light after 5-10 minutes. Subsequently, ethyl difluoroiodoacetate (0.4 mmol) was added into the reaction tube, and reacted for two hours, and the reaction progress was monitored by TLC and GC-MS. After the reaction was over, the reaction tube was taken out, and the reaction solution was removed under reduced pressure using a rotary evaporator to remove volatile solvents, and ethyl acetate / petroleum ether was used as eluent, and purified by column chromatography to obtain monofluoroolefins (76% yield, E / Z=97:3).
[0059]1H NMR (400MHz, CDCl3) δ8.05-7.94 (m, 2H), 7.63-7.49 (m, 3H), 7.44...
Embodiment 2
[0061]
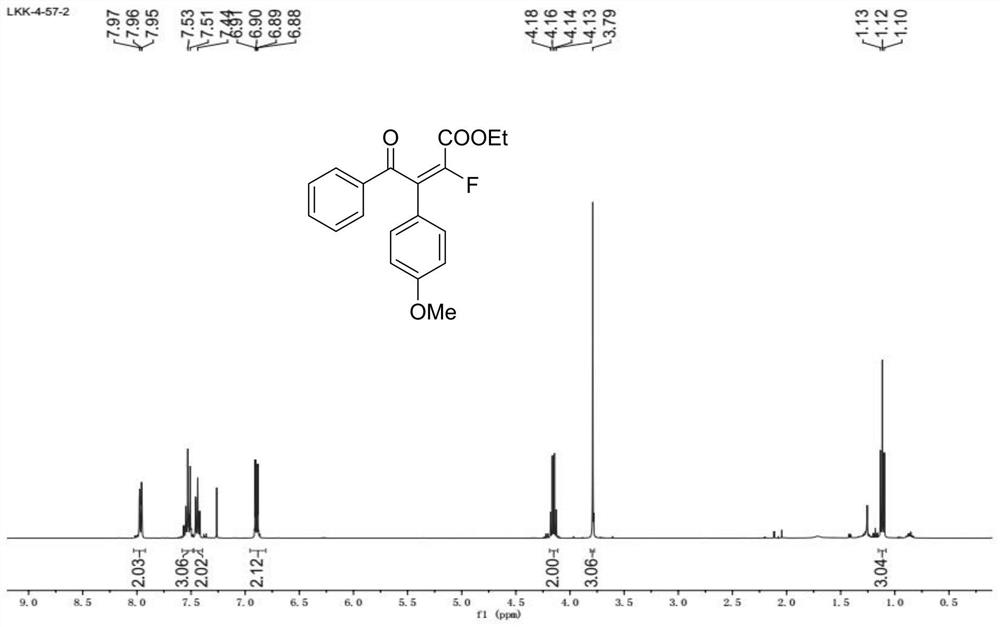
[0062] In the glove box, accurately weigh 2-(4-methoxyphenyl)acetophenone (0.2mmol) and add it to a Schneck reaction tube with a stirring bar, dissolve it in tetrahydrofuran, plug it with a rubber stopper and remove it. glove box. The reaction tube was stirred at -20° C., and then sodium bis(trimethylsilyl)amide (0.32 mmol) was added to the reaction tube with a syringe. Turn on the LED blue light after 5-10 minutes. Subsequently, ethyl difluoroiodoacetate (0.4 mmol) was added into the reaction tube, and reacted for two hours, and the reaction progress was monitored by TLC and GC-MS. After the reaction was over, the reaction tube was taken out, and the reaction solution was removed under reduced pressure using a rotary evaporator to remove volatile solvents, and ethyl acetate / petroleum ether was used as eluent, and purified by column chromatography to obtain monofluoroolefins (67% yield, E / Z=98:2).
[0063] 1 H NMR (400MHz, CDCl3) δ8.03-7.92 (m, 2H), 7.58-7.48 (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com