Application of corydalis saxicola and preparation thereof in preparing medicines for treating non-alcoholic fatty liver diseases
A fatty liver disease, non-alcoholic technology, applied in the field of medicine, can solve the problems of no effective drug treatment and complex pathogenesis, and achieve the effect of expanding clinical indications, preventing liver damage, improving biochemical indicators and imaging evaluation results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 Preparation of total alkaloids of Litectin
[0035] (1) Take 30kg of Yanhuanglian decoction pieces, add 10 times the volume concentration of 75% ethanol and soak for 1 hour;
[0036] (2) Then heat and reflux for extraction three times, each time for 2 hours, and combine the extracts;
[0037] (3) Recover as much ethanol as possible and concentrate to 1:1.3 (Rectinus rhizome W: Concentrate V) to obtain a concentrate;
[0038] (4) Take the above concentrated solution and add 1% hydrochloric acid which is 4 times the amount of the raw material (Rectinus root decoction pieces), put it in an acid-resistant multifunctional extraction tank and heat and reflux for extraction for 1 hour;
[0039] (5) Adjust the extract with 40% sodium hydroxide and keep the pH value to 8, then concentrate to a relative density of 1.06-1.08 (60°C), refrigerate the concentrate, let it stand for 48 hours, and filter; filter the precipitate for use Heat 1% hydrochloric acid 16 times the ...
Embodiment 2
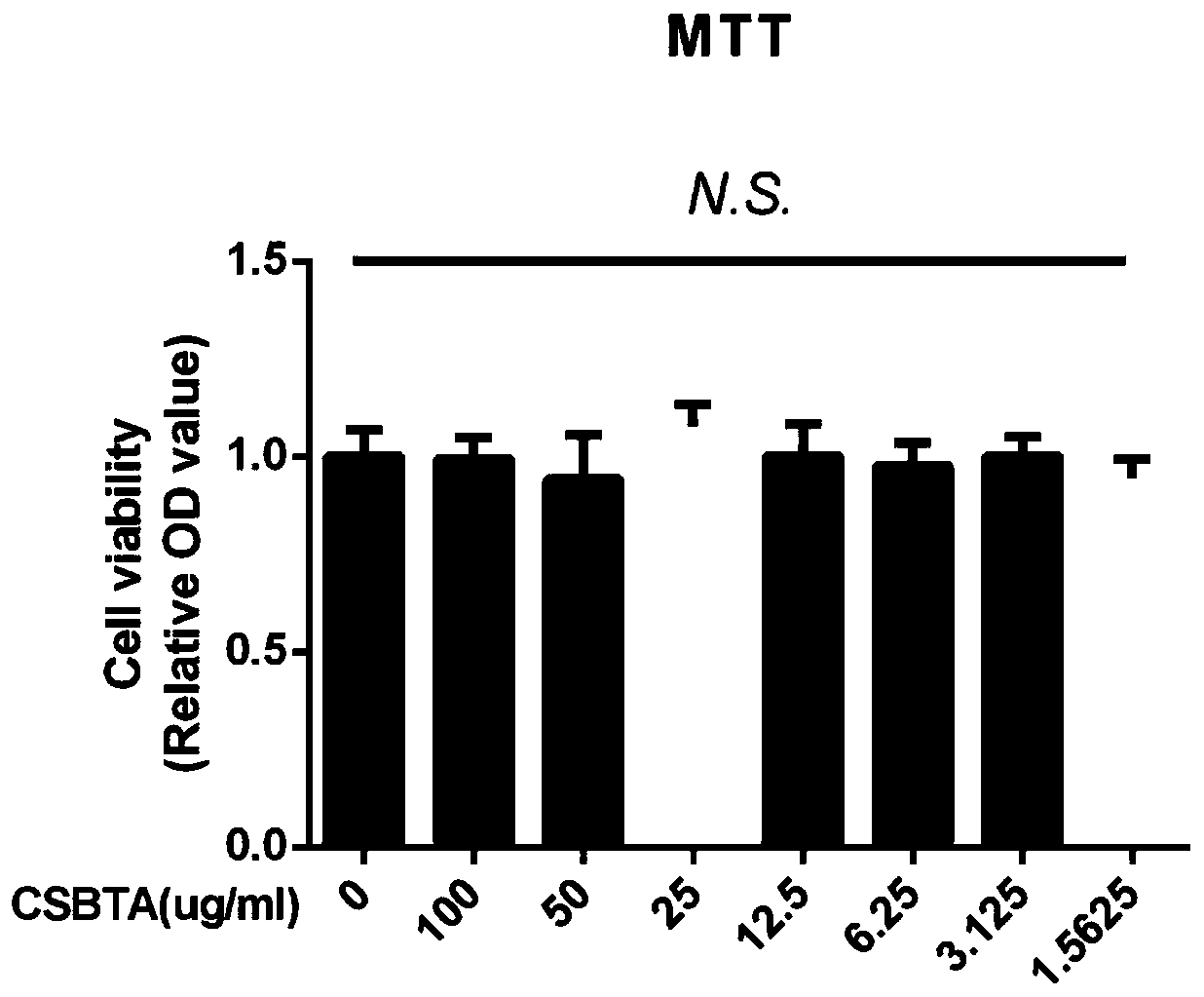
[0043] Example 2 Observation of curative effect of Yanhuanglian total alkaloid capsules on cell experiments.
[0044] 1. Test drug: the total alkaloid extract (CSBTA) of Litectinus chinensis prepared in Example 1.
[0045] 2. Cytotoxicity experiment
[0046] 1.1 Experimental instrument: MULTSKAN Sky full-wavelength microplate reader (Thermo Scientific, USA)
[0047] 1.2 Reagents: MTT cell proliferation and cytotoxicity detection kit (article number: KGA321, Jiangsu Kaiji Biotechnology Co., Ltd.); total alkaloids of Litectinum prepared in Example 1;
[0048] 1.3 Experimental method: Add 100 µL / well of cells in a 96-well plate (about 1×10 4 ), set at 37℃, 5%CO 2 Cell culture incubator for 24 hours. Aspirate the medium of all wells, use the blank medium without adding serum to configure CSTBA (100ug / mL) solution, dilute it to seven concentrations of 50, 25, 12.5 and so on, and mix the blank medium with Each concentration of medium was added to a 96-well plate and placed at 3...
Embodiment 3
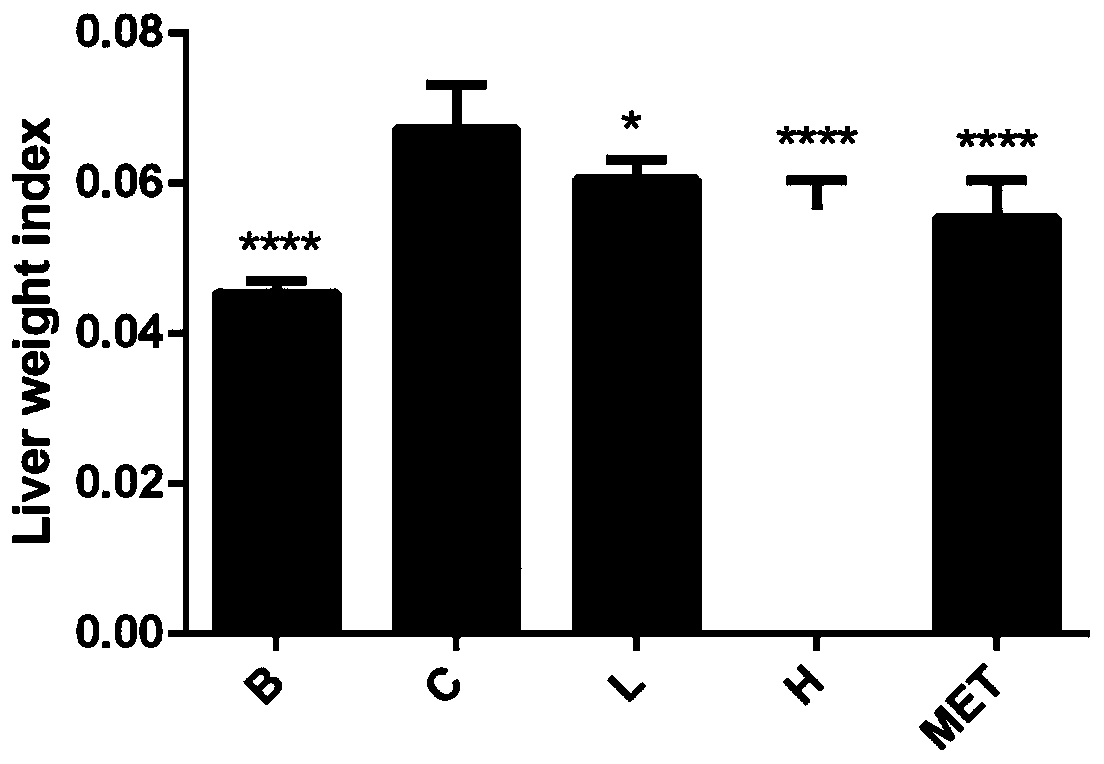
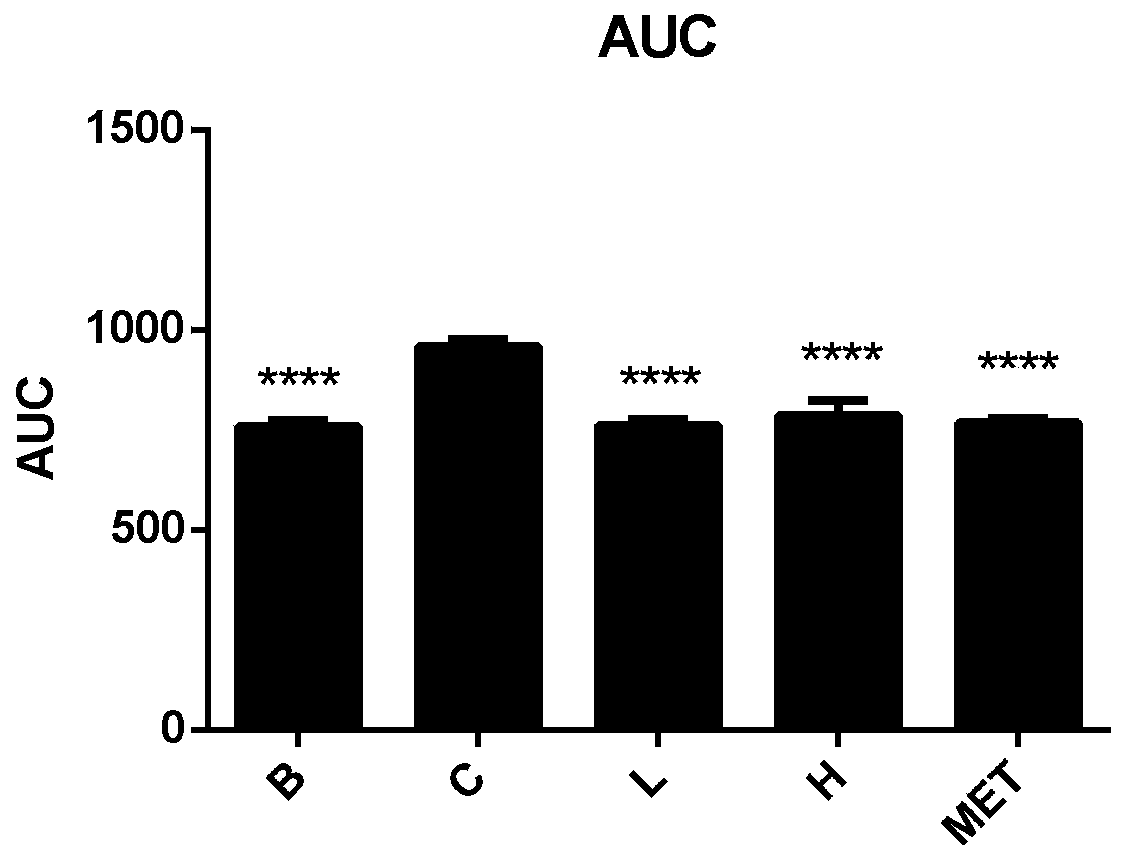
[0050] Example 3 Observation of the curative effect of total alkaloids of Litectinum chinensis on hypolipidemic effect of hyperlipidemia model rats
[0051] 1. Experimental modeling
[0052] 1.1 Experimental animals: male C57BL / 6 black mice, 4 weeks old, weighing about 19g, purchased from the Comparative Medicine Center of Yangzhou University.
[0053] 1.2 Feed: 60% high-fat feed, fructose, and normal feed were purchased from Nantong Trophy Feed Technology Co., Ltd.
[0054] 1.3 Reagents: the total alkaloids extract of Litectinus rhizome prepared in Example 1.
[0055] 1.4 Experimental method: Take 35 male C57BL / 6 small black mice, 4 weeks old, after one week of adaptive feeding, divide the mice into two initial groups randomly, and feed them with normal diet or high-fat (60% fat) high-sugar Diet (20% fructose). After feeding for 10 weeks, the mice were randomly divided into model groups, total alkaloids of Litectin (25mg / kg), total alkaloids of Litectin (100mg / kg), metformin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com