A kind of chiral gamma-butyrolactone derivative and its synthesis method and application
A technology for butyrolactone and derivatives, applied in the field of optically active γ-butyrolactone derivatives and their synthesis, can solve the problems of many steps in the synthesis process, unstable raw materials, inconvenient operation, etc., and achieve fewer reaction steps and better Anti-cancer effect with low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
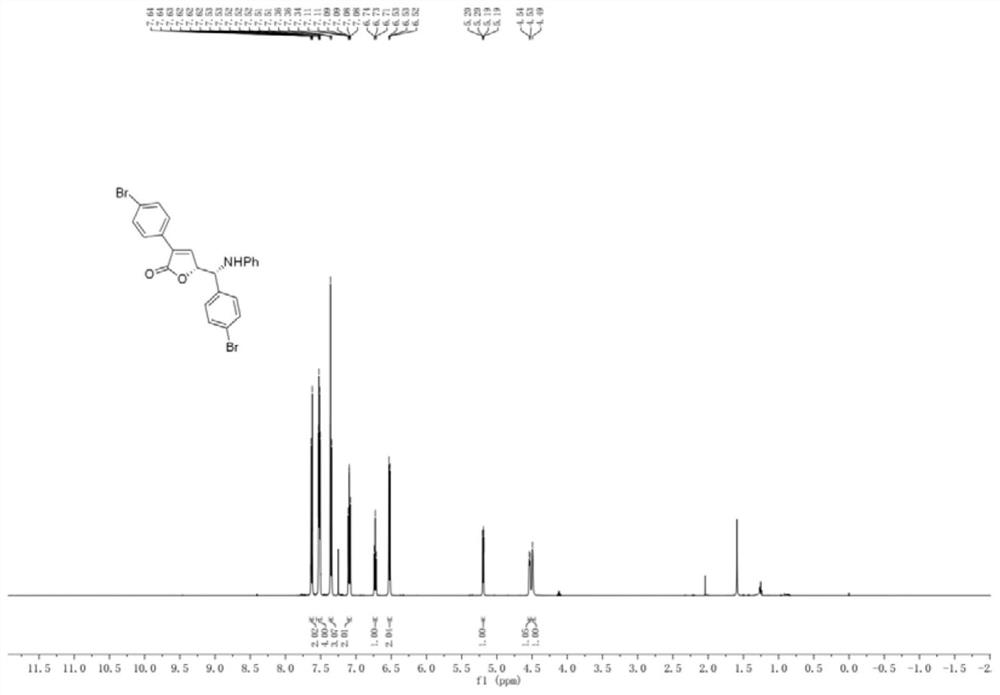
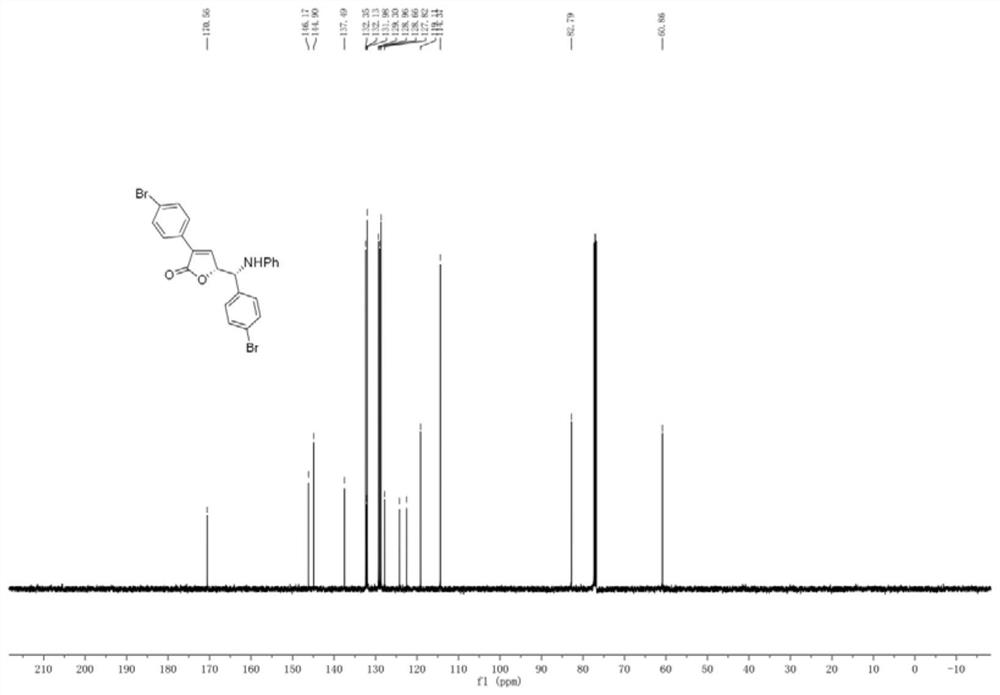
[0055] The preparation of embodiment 1 compound 3b1
[0056]
[0057] Rh 2 (esp) 2 (0.008mmol), chiral phosphoric acid (0.02mmol), 3-p-bromophenylcyclopropene carboxylic acid (0.36mmol), and p-bromobenzylideneaniline (0.2mmol) were dissolved in a test tube containing 2.5mL tetrahydrofuran, and Stir at a specific temperature (25° C.) and react for 96 hours. After the imine reaction is complete, the solvent is removed by rotary evaporation under reduced pressure to obtain a crude product. The crude product was subjected to column chromatography (ethyl acetate:petroleum ether=1:10-1:3) to obtain a pure product. Yield 91%, dr>95:5, er:93:7.
[0058] 1 H NMR (500MHz, CDCl 3 )δ7.65–7.61(m,2H),7.55–7.49(m,4H),7.38–7.33(m,3H),7.12–7.06(m,2H),6.73(dd,J 1 =J 2 =7.4Hz,1H),6.55–6.50(m,2H),5.19(dd,J=6.4,1.8Hz,1H),5.19(dd,J=6.4,1.8Hz,1H),4.53(d,J= 6.1Hz, 1H), 4.53(d, J=6.1Hz, 1H), 4.49(s, 1H), 4.49(s, 1H). 13 C NMR (125MHz, CDCl 3 )δ170.6,146.2,144.9,137.5,132.4,132.1,132.0,129...
Embodiment 2
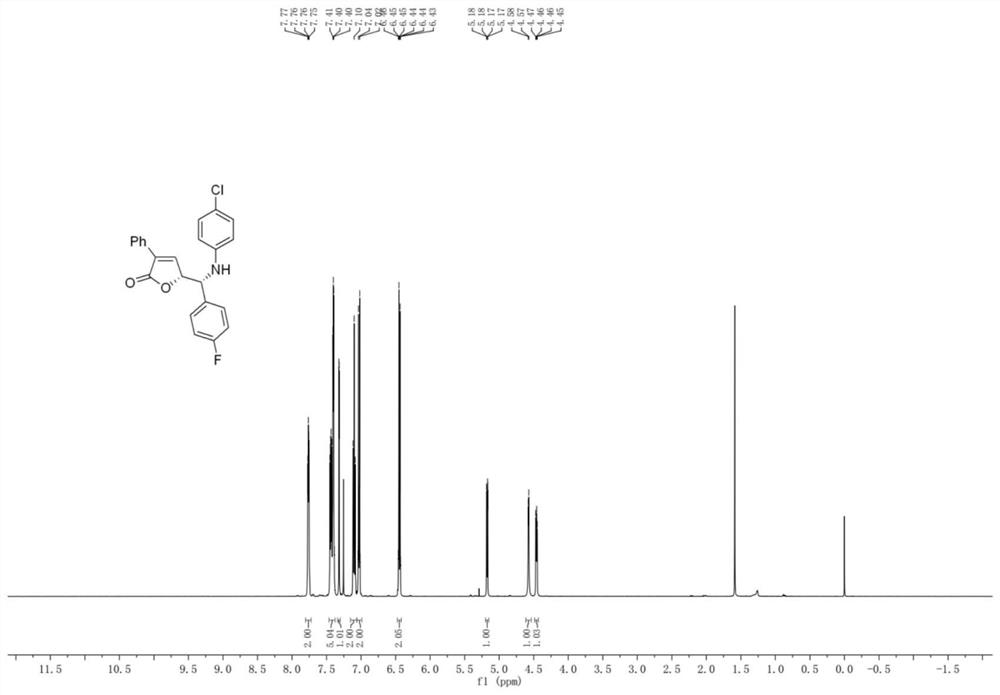
[0059] The preparation of embodiment 2 compound 3aq
[0060]
[0061] Rh 2 (esp) 2 (0.008mmol), chiral phosphoric acid (0.02mmol), 3-phenylcyclopropene carboxylic acid (0.36mmol), and p-fluorobenzylidene p-chloroaniline (0.2mmol) were dissolved in a test tube containing 2.5mL tetrahydrofuran, and Stir at a specific temperature (25° C.) and react for 96 hours. After the imine reaction is complete, the solvent is removed by rotary evaporation under reduced pressure to obtain a crude product. The crude product was subjected to column chromatography (ethyl acetate:petroleum ether=1:10-1:3) to obtain a pure product. Yield 80%, dr: 94:6, er: 95:5.
[0062] 1 H NMR (500MHz, CDCl 3 )δ7.76(dd,J 1 =J 2 =6.6,3.0Hz,2H),7.46–7.37(m,5H),7.32(d,J=1.8Hz,1H),7.10(dd,J 1 =J 2 =8.5Hz,2H),7.05–7.01(m,2H),6.47–6.42(m,2H),5.17(dd,J=6.9,1.7Hz,1H),4.57(d,J=3.9Hz,1H) ,4.46(dd,J=6.8,4.2Hz,1H). 13 C NMR (125MHz, CDCl 3 )δ170.8, 162.7 (d, J = 247.9Hz), 144.9, 144.4, 133.8 (d, J = 3.2Hz), 1...
Embodiment 3
[0063] The preparation of embodiment 3 compound 3a1
[0064]
[0065] Rh 2 (esp) 2 (0.008mmol), chiral phosphoric acid (0.02mmol), 3-phenylcyclopropene carboxylic acid (0.36mmol), benzylidene p-bromoaniline (0.2mmol) were dissolved in a test tube containing 2.5mL tetrahydrofuran, at a specific temperature Stir at (25° C.) and react for 96 hours. After the imine reaction is complete, the solvent is removed by rotary evaporation under reduced pressure to obtain a crude product. The crude product was subjected to column chromatography (ethyl acetate:petroleum ether=1:10-1:3) to obtain a pure product. Yield 89%, dr:95:5, er:94.5:5.5.
[0066] 1 H NMR (400MHz, CDCl 3 )δ7.75(dd,J 1 =J 2 =6.4,2.7Hz,2H),7.49–7.28(m,9H),7.15(d,J=8.7Hz,2H),6.41(d,J=8.7Hz,2H),5.19(dd,J=7.1, 1.2Hz, 1H), 4.61(d, J=3.5Hz, 1H), 4.44(dd, J=6.9, 4.1Hz, 1H). 13 C NMR (100MHz, CDCl 3 )δ170.9,145.6,144.8,138.0,133.0,131.9,129.7,129.3,129.1,128.8,127.21,127.15,116.0,110.6,83.0,61.9.HRMS(ESI)m / z:calcd....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com