Novel fusarisetin herbicide derived from marine fungus, preparation method and application
A technology of marine fungi and herbicides, applied in the directions of herbicides and algicides, botany equipment and methods, biochemical equipment and methods, etc., which can solve the problems of undisclosed herbicide effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
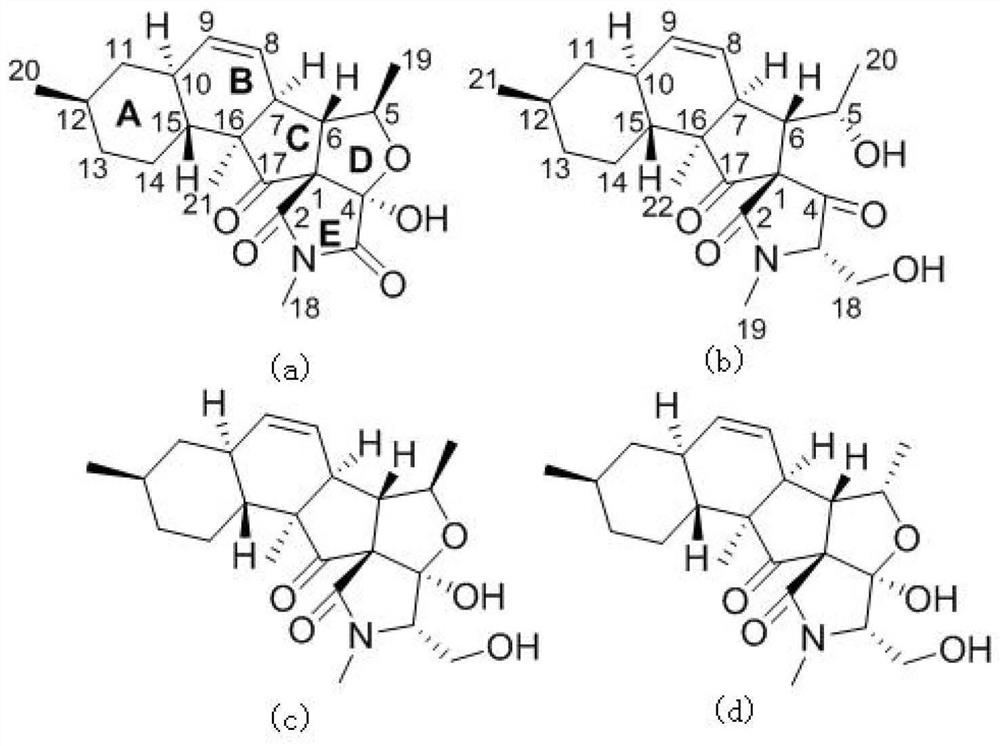
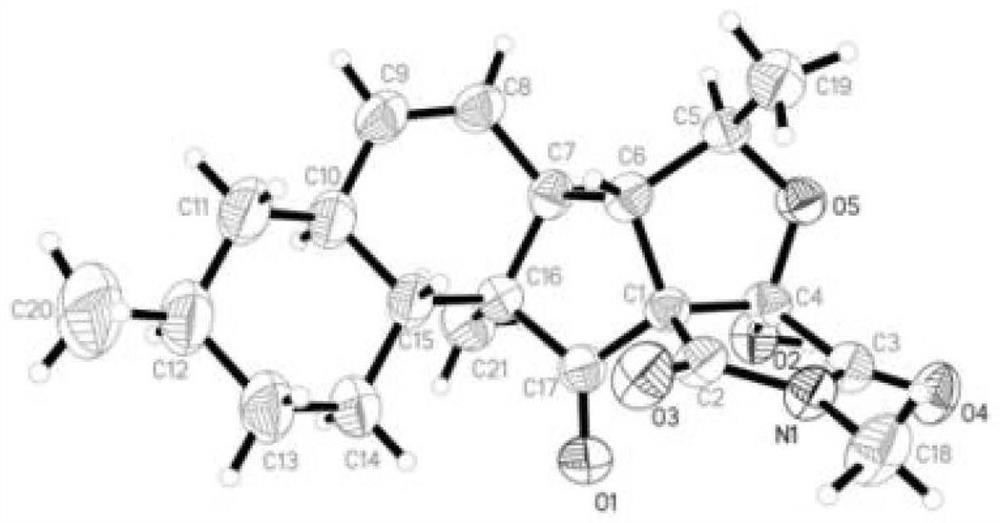
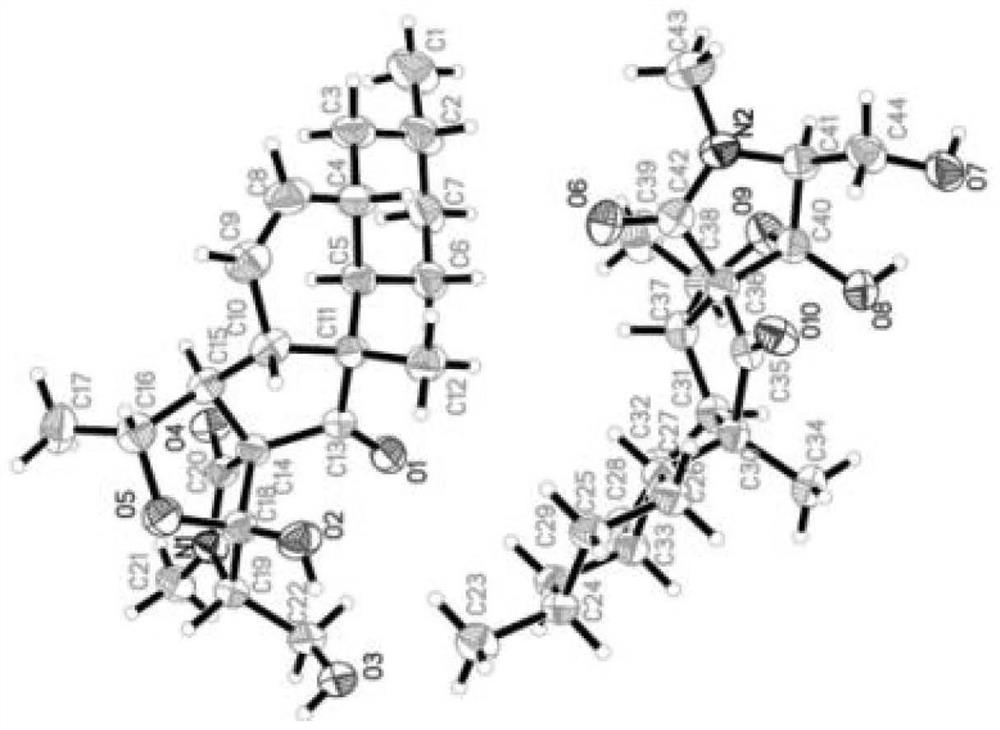
[0041] In this example, the structure of fusarisetins C–D (compound 1–2) was determined by NMR, mass spectrometry, infrared, ultraviolet, vibrational circular dichroism and X-Ray single crystal diffraction. Compound 2 is the first reported compound with 6 / 6 decahydronaphthalene The spectral data of fusarisetin C–D (compound 1–2), a fusarisetin compound chimeric with a 5 / 5 bicyclic ring, are as follows:
[0042] Fusarisetin C(1): colorless crystals; [α] 20 D +15.0 (concentration 0.25, methanol); UV (methanol) λ max (logε)202(3.42)nm; hydrogen spectrum and carbon spectrum data are shown in Table 1; high resolution mass spectrum m / z 372.1825[M -H] - (C 21 h 27 NO 5 Theoretical value, 372.1816);
[0043] Fusarisetin D(2): colorless oil; [α] 20 D -9.2 (concentration 0.13, methanol); UV (methanol) λ max (logε)200(3.19)nm; hydrogen spectrum and carbon spectrum data are shown in Table 2; high resolution mass spectrum m / z 390.2278[M+ H] + (C 22 h 32 NO 5 Theoretical value...
Embodiment 2
[0050] In this example, Amaranthus retroflexus and lettuce seeds were used as active targets to test their herbicidal activity. The results are shown in Table 3. The specific process is as follows:
[0051]First, sterilize the seeds of dicotyledonous plants such as Amaranthus retroflexus with 3%-5% sodium hypochlorite (NaClO) solution for 5-15 minutes, rinse with sterile water several times to remove sodium hypochlorite, and then dissolve the compound in methanol to prepare 0.2 mg / mL and 0.05 mg / mL solution, then put filter paper in a 24-well plate, add 200 μL compound solution to each well, after the solvent evaporates, add 200 μL sterile water to each well, seal it and put it in a light incubator at 28 ° C for 12 h Light, 12h dark culture, count germination rate and rhizome growth after 4 days.
[0052] Table 3: Compounds 1–4 (200 μg / mL) inhibited the growth of Amaranthus retroflexus and lettuce root shoots
[0053]
[0054] Among them, "gp" glyphosate. Length<2.0mm is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com