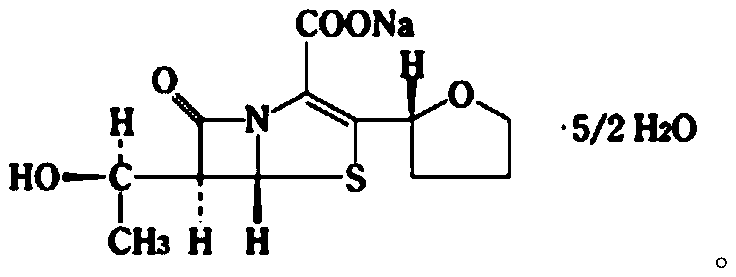
Faropenem sodium particles and preparation method thereof
A technology for faropenem sodium and granules, which is applied in the field of faropenem sodium granules and its preparation, and can solve the problems of simple preparation process, small toxic and side effects, and low polymer content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
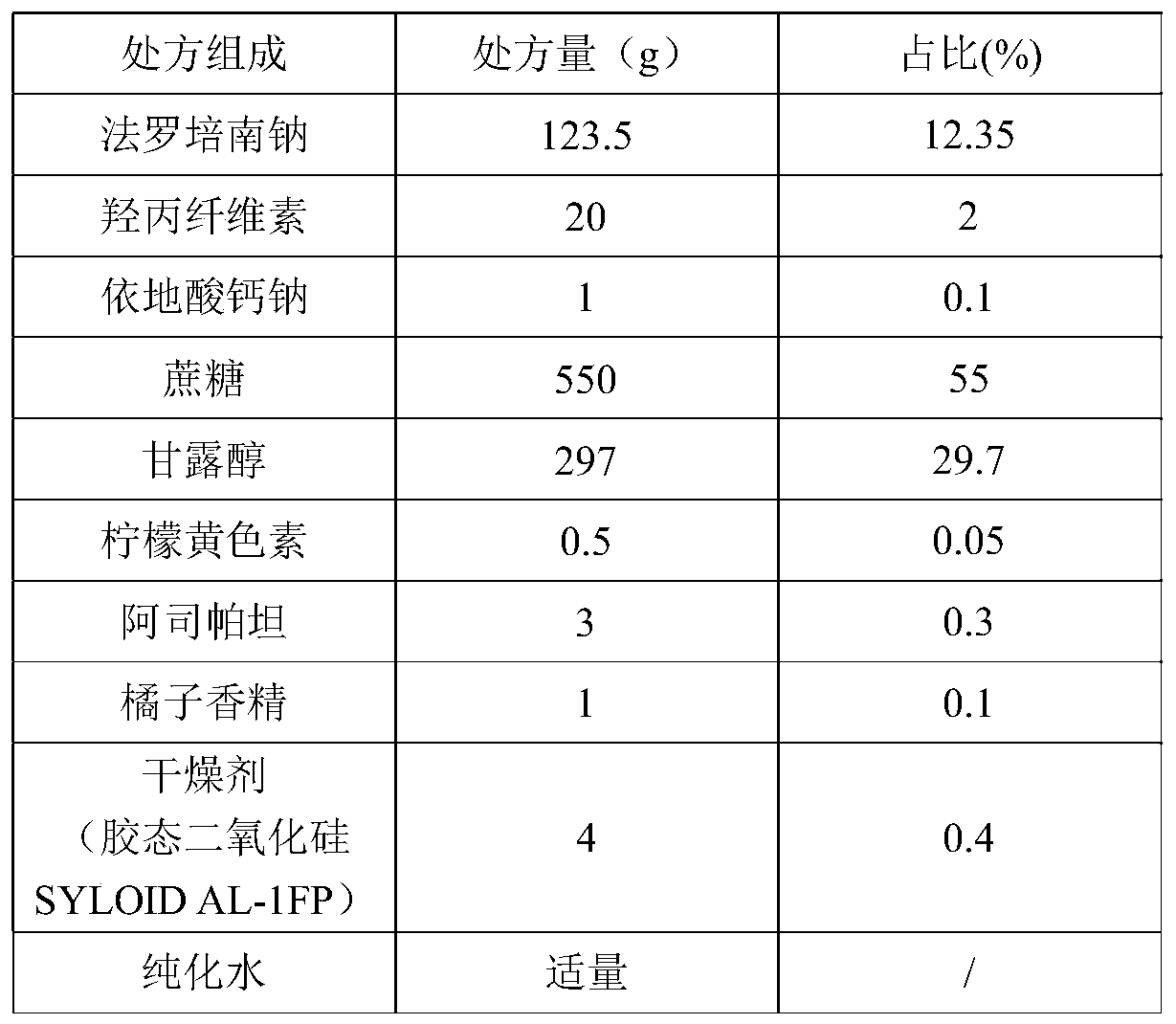
[0027]
[0028] Preparation Process:
[0029] Mix faropenem sodium passed through a 80-mesh sieve with sucrose powder, mannitol, hyprolose, edetate calcium sodium, aspartame, and flavors respectively passed through a 100-mesh sieve, and dissolve tartrazine in the purified Use water as a wetting agent, use a fluidized bed for granulation, dry at 40±5°C until the water content is within 2%, sieve with a 10-mesh sieve and an 80-mesh sieve, and take 10-80 mesh granules for later use; Another appropriate amount of fine powder under 80 mesh (equivalent to 5% of the total prescription amount) is added to the desiccant passed through a 120 mesh sieve and mixed, and then the prescription amount of 10-80 mesh granules is added to mix, the filling amount is measured, and packaged.
Embodiment 2
[0031] prescription composition Prescription volume (g) Proportion (%) Faropenem Sodium 123.5 12.35 Hypromellose 20 2 Edetate Calcium Sodium 1 0.1 sucrose 548 54.8 Mannitol 297 29.7 Lemon yellow pigment 0.5 0.05 aspartame 3 0.3 orange flavor 1 0.1 diatomite 6 0.6 purified water Appropriate amount /
[0032] Preparation Process:
[0033] Mix faropenem sodium passed through a 80-mesh sieve with sucrose powder, mannitol, hyprolose, edetate calcium sodium, aspartame, and flavors respectively passed through a 100-mesh sieve, and dissolve tartrazine in the purified Use water as a wetting agent, use a fluidized bed for granulation, dry at 40±5°C until the water content is within 2%, sieve with a 10-mesh sieve and an 80-mesh sieve, and take 10-80 mesh granules for later use; Another appropriate amount of fine powder under 80 mesh (equivalent to 5% of the total prescription amount) is added to the d...
Embodiment 3
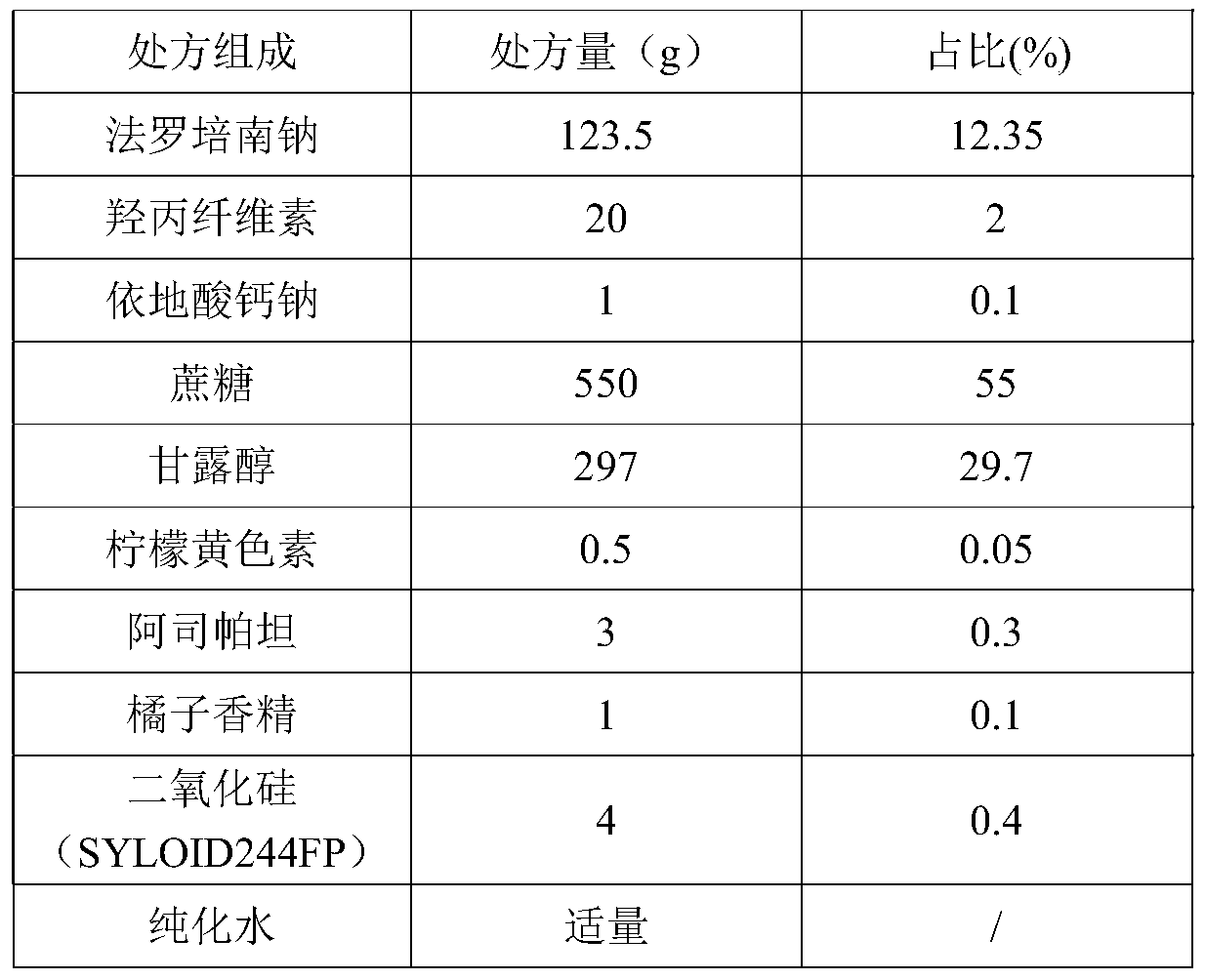
[0035]
[0036] Preparation Process:
[0037] Mix faropenem sodium passed through a 80-mesh sieve with sucrose powder, mannitol, hyprolose, edetate calcium sodium, aspartame, and flavors respectively passed through a 100-mesh sieve, and dissolve tartrazine in the purified Use water as a wetting agent, use a fluidized bed for granulation, dry at 40±5°C until the water content is within 2%, sieve with a 10-mesh sieve and an 80-mesh sieve, and take 10-80 mesh granules for later use; Another appropriate amount of fine powder under 80 mesh (equivalent to 5% of the total prescription amount) is added to the desiccant passed through a 120 mesh sieve and mixed, and then the prescription amount of 10-80 mesh granules is added to mix, the filling amount is measured, and packaged.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com