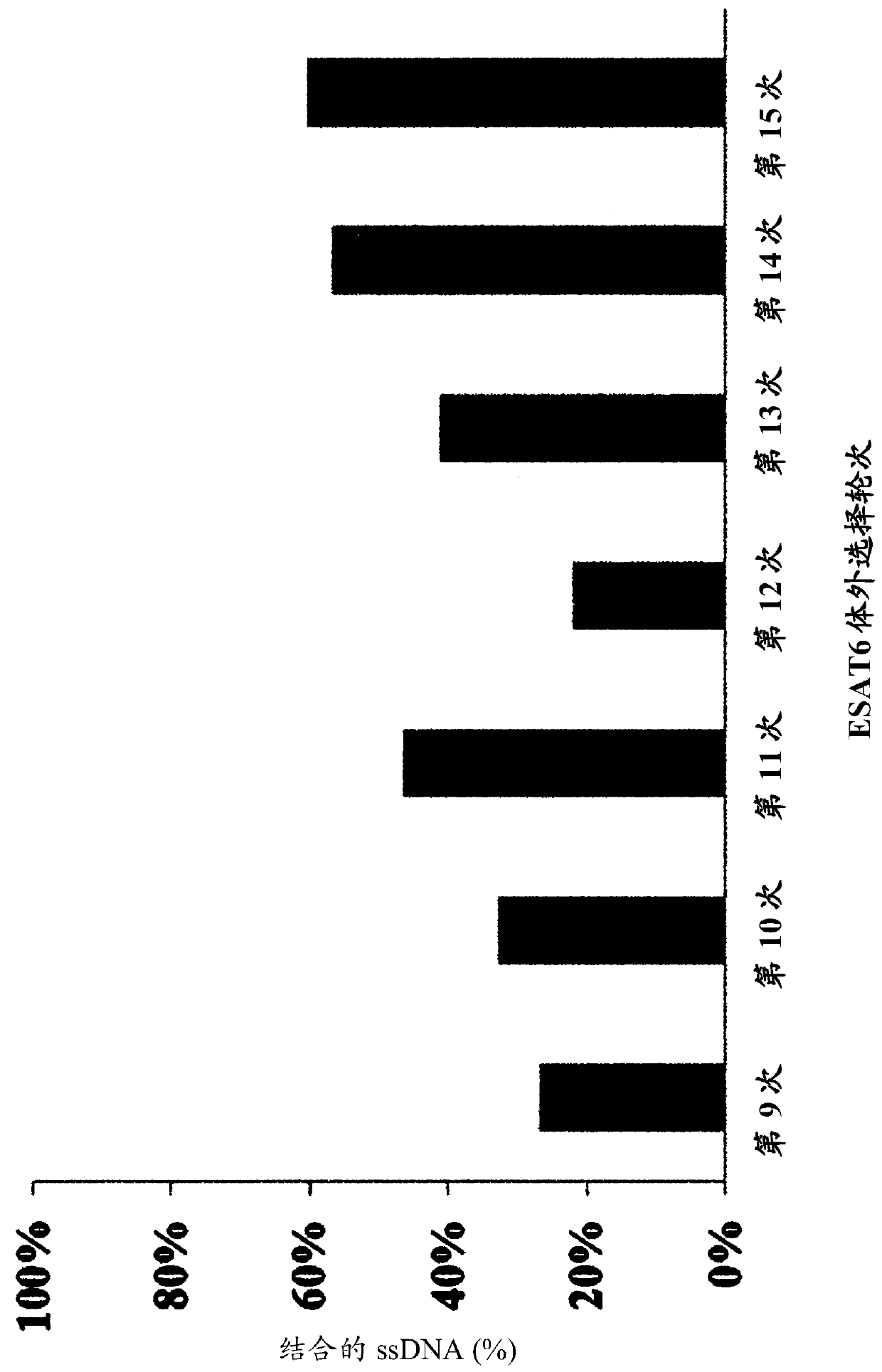
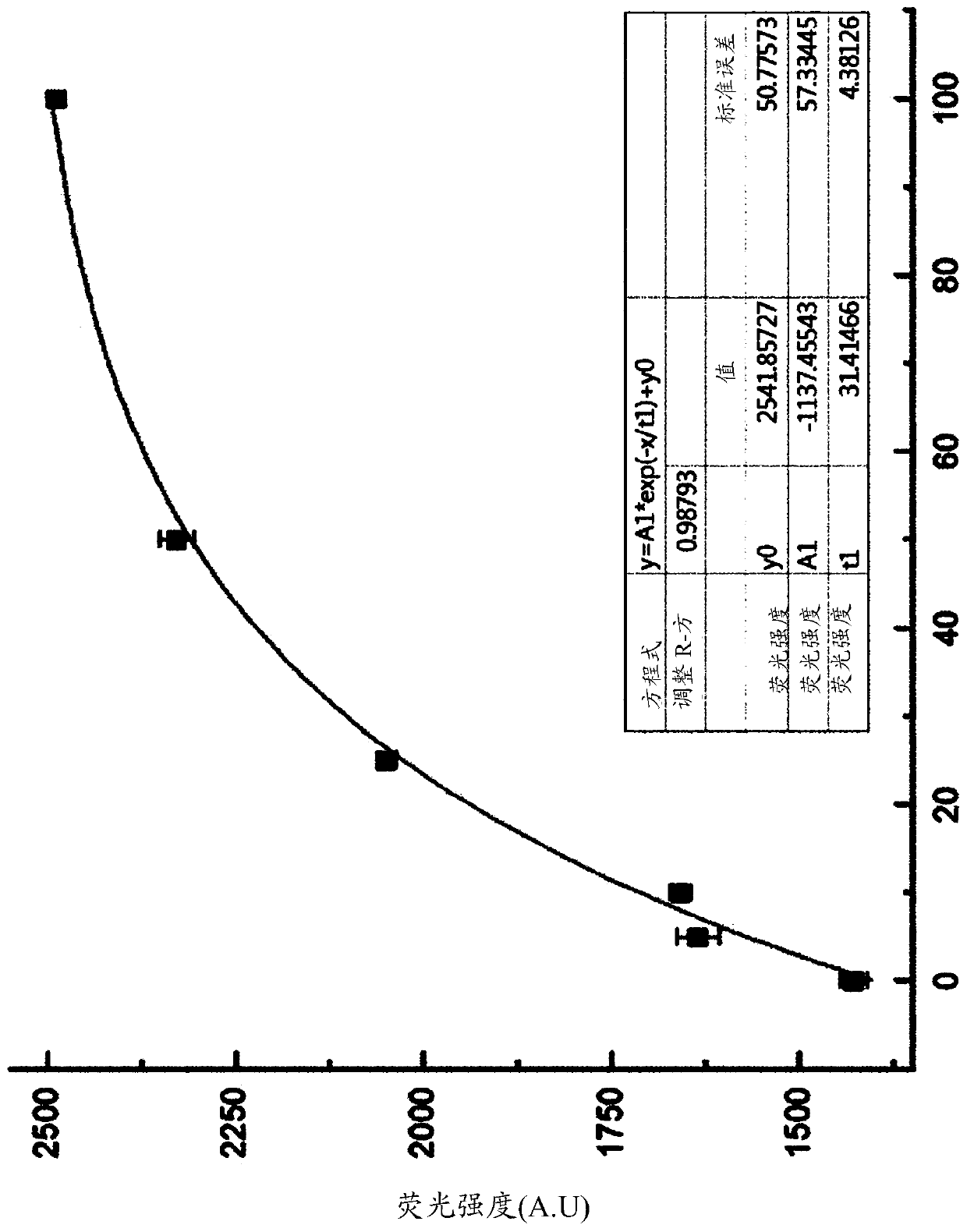
DNA aptamer binding specifically to esat6 and use thereof
A DNA aptamer and specific technology, applied in the field of DNA aptamers, can solve problems such as lack of stability and reduced sensitivity, and achieve excellent stability, high exclusivity, and excellent binding ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
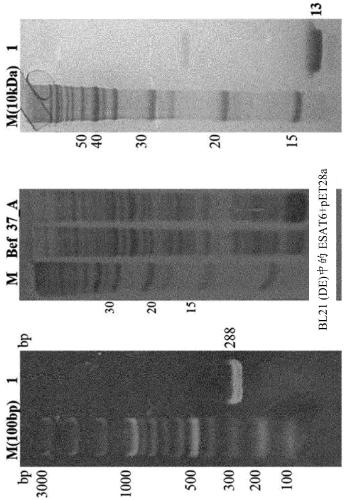
[0049] Embodiment 1. ESAT6 gene cloning
[0050] To amplify the gene for the 6 kDa early secretory antigen target (ESAT6), a forward primer including the BamH1 restriction enzyme recognition sequence [5'-CCC GGATCC ATG ACA GAG CAG CAG TGG AAT TT3'( SEQ ID NO:1)], the reverse primer includes the Hind3 restriction enzyme recognition sequence [5'CCC CCA AGC TTC TAT GCGAAC ATC CCA GTG A3'(SEQ ID NO:2)].
[0051] Genomic DNA of M. tuberculosis H37Rv was used as a template for gene amplification and was amplified by polymerase chain reaction (PCR) using i-pfu polymerase. Each process of PCR is as follows: 1) Incubate at 98°C for 20 seconds, as a process of denaturing double-stranded DNA into a template; 2) Incubate at 57°C for 20 seconds, as a process of annealing the template with primers; 3) Incubate at 72°C 30 seconds, as a process of extending new strands, and a cycle of repeating these processes 30 times.
[0052] The amplified ESAT6 gene was cloned into a (His)6-tag-contai...
Embodiment 2
[0053] Example 2. Expression of ESAT6 protein
[0054] BL21 (DE3) cells transformed with ESAT6 gene were cultured in Luria Bertani (LB) medium and incubated at 37° C. until the optical density (OD) at UV 600 nm reached 0.563. Thereafter, isopropyl-thio-b-D-galactopyranoside (IPTG) was added to a final concentration of 20 mM to induce the expression of the protein, followed by incubation at 37°C for 4 hours. The expression of the protein was confirmed by SDS PAGE, and the cells were separated from the medium using a centrifuge and washed once with PBS (10 mM sodium phosphate, 150 mM NaCl, pH 8.0) buffer.
Embodiment 3
[0055] Example 3. Purification of ESAT6 protein
[0056] In order to purify ESAT6 protein expressed in bacterial cells BL21(DE3) with high purity, cells were lysed in cell lysis buffer (20 mM Tris, 500 mM NaCl, 0.5 mM β-mercaptoethanol, 5% glycerol, pH 8.0) and then washed with Ultrasound for 15 minutes to rupture. A centrifuge was used at 18,000 rpm for 40 minutes to separate the protein in an aqueous solution from the cells.
[0057] After centrifugation, the pellet was added to and allowed to dissolve in refolding buffer containing 8M urea (8M urea, 20mM Tris-HCl, 500mM NaCl, 15mM imidazole, 0.5mM β-mercaptoethanol, 5% glycerol, pH 8.0) . Separation was performed at 18,000 rpm for 50 minutes using a centrifuge to separate proteins dissolved in an aqueous solution state from the cells.
[0058] Furthermore, in order to obtain high-purity proteins, the binding properties between nickel-nitrilotriacetic acid (Ni-NTA) and (His)6-tagged amino acids were utilized. Specifica...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com