Indole salt-coumarin derivative, and synthesis method and application thereof
A technology of coumarin derivatives and synthesis methods, applied in instruments, analytical materials, organic chemistry, etc., to achieve the effects of good selectivity, simple detection means, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Preparation and characterization of Mito-CI
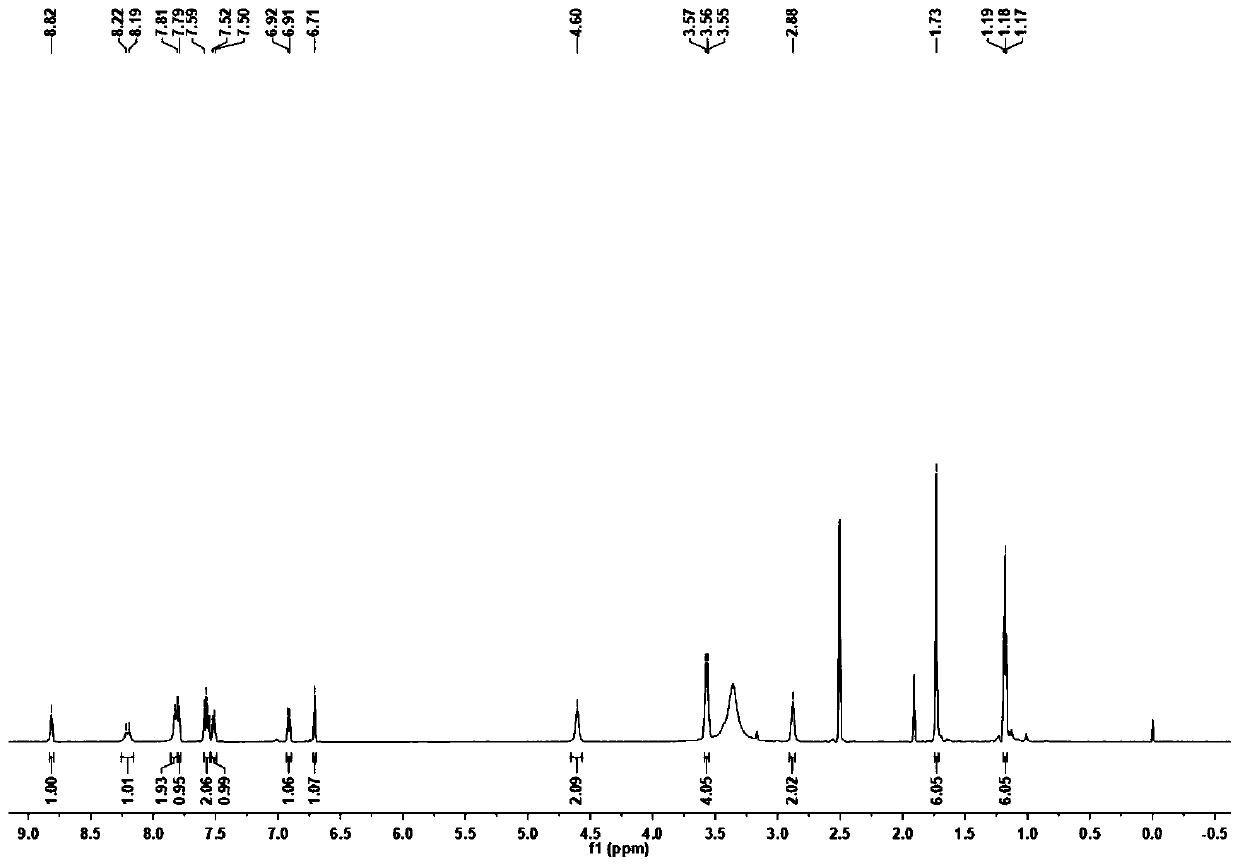
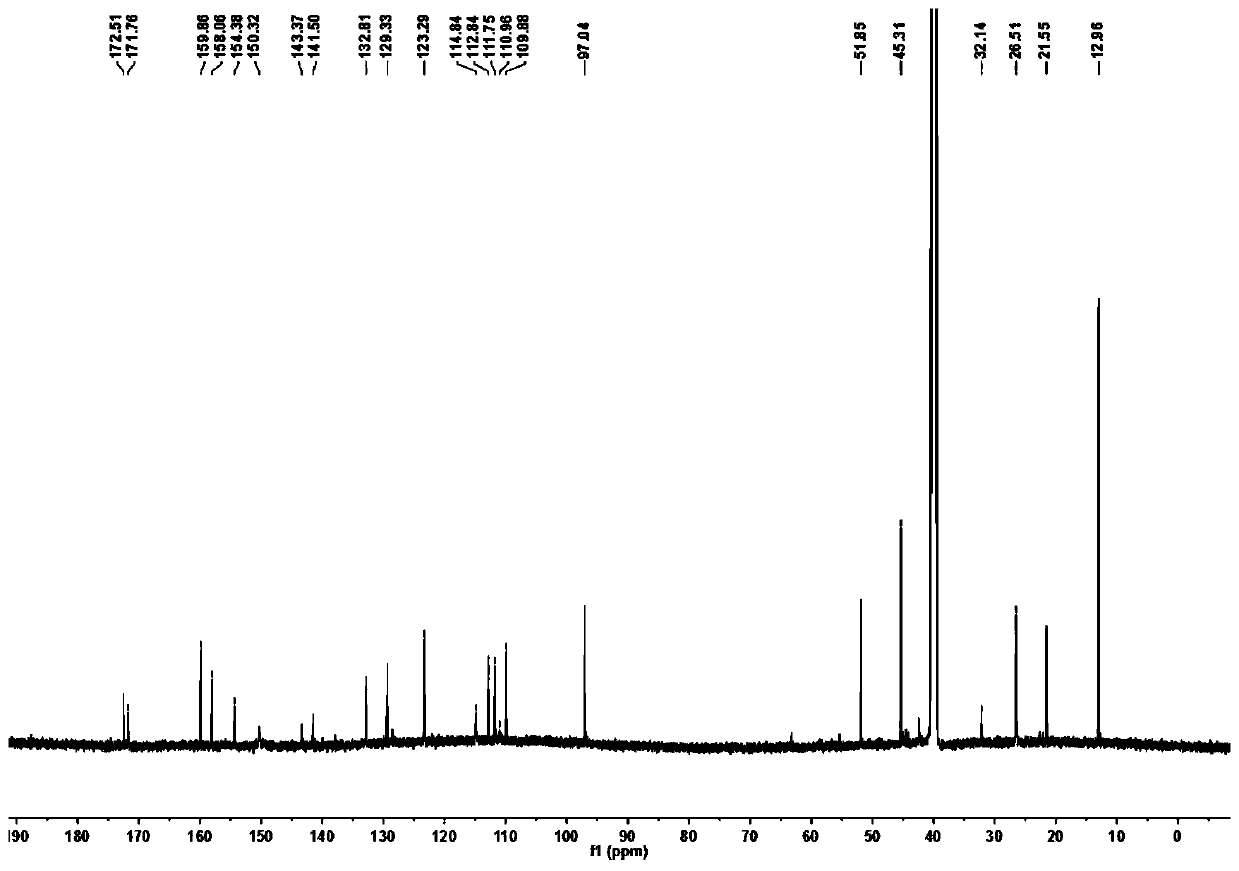
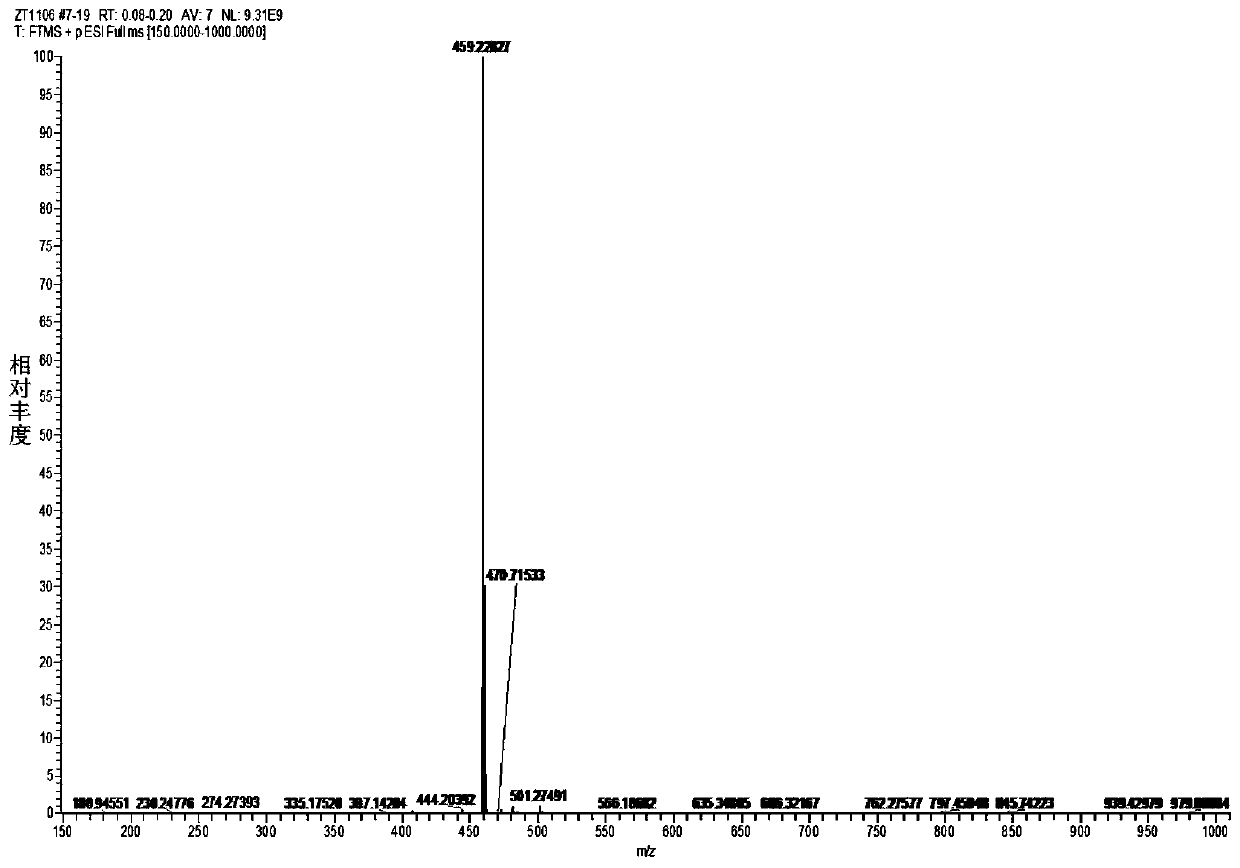
[0041] Diethyl malonate (6.08g, 38mmol), 4-(diethylamino)-2-hydroxybenzaldehyde (3.67g, 19mmol) and piperidine (2.0mL) were mixed with absolute ethanol (60mL), then The mixture was refluxed for 12 hours. After the reaction was completed, the solvent was evaporated under reduced pressure. Next, a mixture of concentrated acetic acid (40 mL) and HCl (40 mL) was added and the reaction was refluxed at 120° C. for 13 hours. Next, after cooling to 25°C, the reaction mixture was poured into 200 ml of water at 0°C. NaOH solution (40%) was added to raise the pH to 5 and a brown precipitate formed immediately. , the mixture was filtered and washed four times with water, dried in vacuo to obtain a yellow-brown solid. (3.92g, Yield: 95%). 1 H NMR (600MHz, DMSO-d 6 )δ7.82(d, J=9.3Hz, 1H), 7.42(d, J=8.8Hz, 1H), 6.68(dd, J=8.8, 2.5Hz, 1H), 6.51(d, J=2.3Hz, 1H), 5.99(d, J=9.3Hz, 1H), 3.42(q, J=7.0Hz, 4H), 1.12(t, J=7.0Hz, 6H). 13 C N...
Embodiment 2
[0046] Prepare a PBS buffer solution with a pH of 7.4 and a concentration of 10 mM, prepare a 2 mM Mito-CI DMSO solution, and prepare a 2 mM sulfur dioxide aqueous solution; take 2 mL of pure PBS buffer solution and 10 μL Mito-CI DMSO solution into a fluorescence cuvette, and take The aqueous solution of sulfur dioxide was gradually added to the cuvette with a micro-injector, and was detected on the fluorescence spectrophotometer while adding the sample. With the addition of sulfur dioxide, the fluorescence intensity at 481nm gradually increased, and the fluorescence intensity at 655nm gradually decreased. Fluorescence emission map see Figure 4 .
Embodiment 3
[0048] Prepare PBS buffer solution with pH=7.4 and concentration of 10mM, prepare 2mM Mito-CI DMSO solution, prepare 2mM sulfur dioxide aqueous solution, prepare 2mM formaldehyde aqueous solution, take 2mL pure PBS buffer solution, 10μL Mito-CI DMSO solution and add to a fluorescent Add 100 μL of sulfur dioxide aqueous solution to the cuvette to make Mito-CI-SO 3 2- DMSO solution, take the aqueous solution of formaldehyde, gradually add it to the cuvette with a micro-injector, and detect it on the fluorescence spectrophotometer while adding the sample. The fluorescence intensity gradually increased. Fluorescence emission map see Figure 5 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com