CO2 complete methanation catalyst as well as preparation and application thereof
A technology for complete methanation and catalyst, applied in the field of carbon dioxide methanation catalyst and preparation thereof, can solve the problems of complex active components, high cost, unsuitability for wide-scale promotion, etc., and achieves simple preparation method, energy saving, and use mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
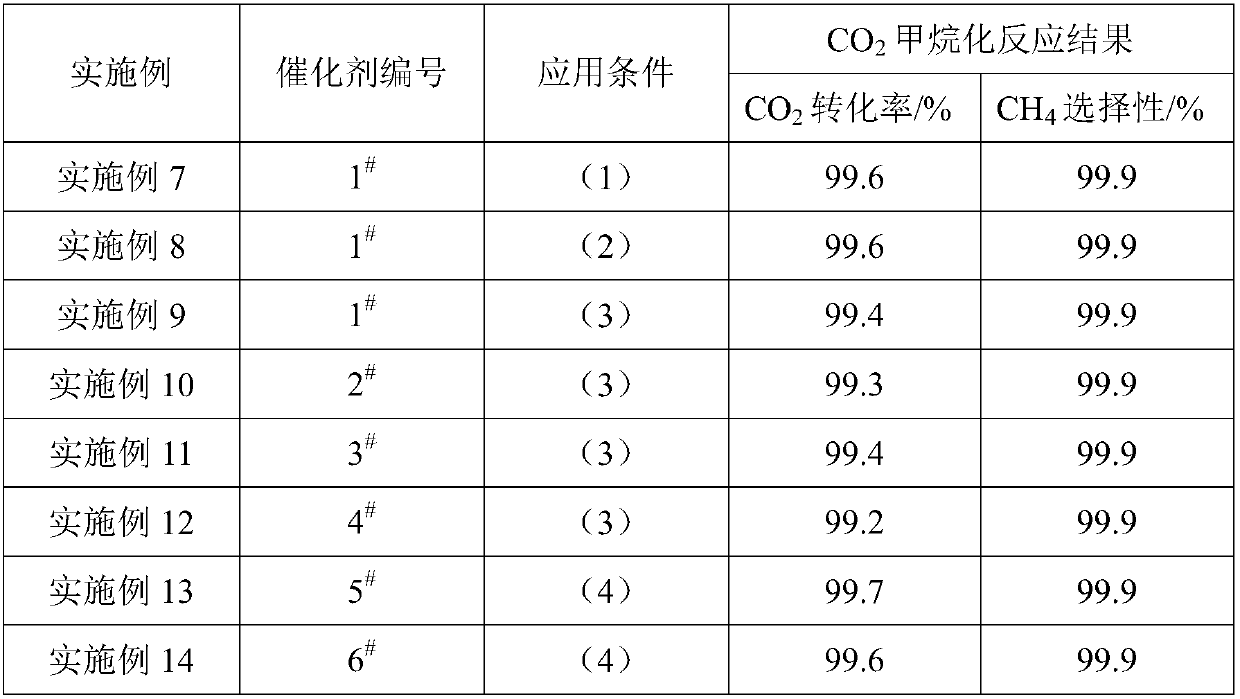
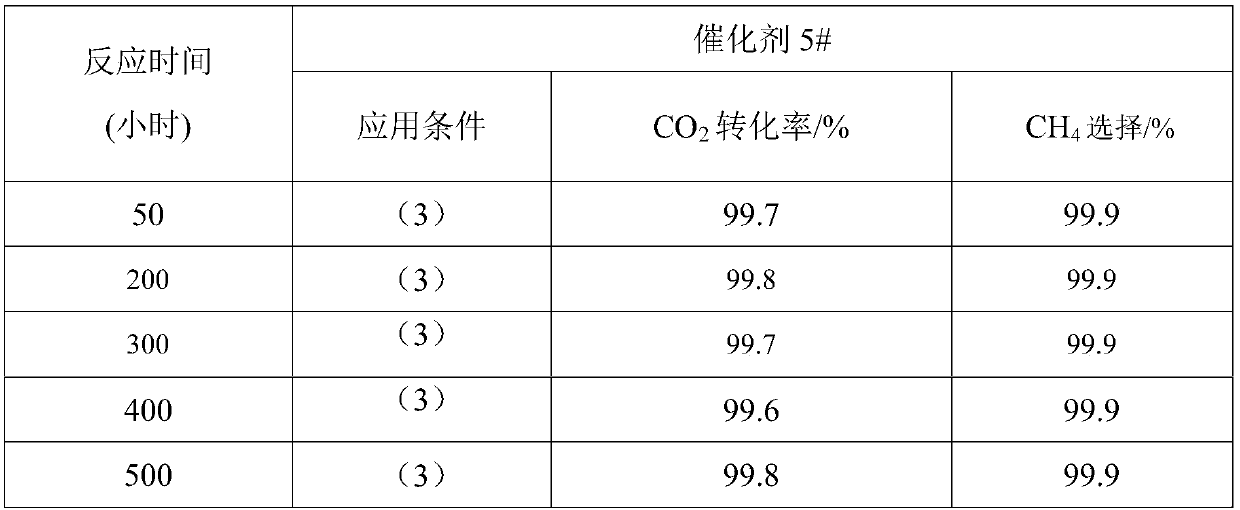
[0030] Weigh 9.7gNi(NO 3 ) 2 ·6H 2 O, 0.97gLa(NO 3 ) 3 ·6H 2 O, 52.2gAl(NO 3 ) 3 9H 2 O was dissolved in 100mL deionized water and stirred to dissolve into a salt solution, and another 50g (NH4 ) 2 CO 3 Solid solution 150mL deionized water is made into alkaline solution. At room temperature, the two solutions prepared above are subjected to co-precipitation reaction by co-current co-precipitation method. During the precipitation process, the pH value of the mother solution is controlled to be 8. After the precipitation is completed, continue to stir and age 3h, then the precipitated product was washed with deionized water, and then dried at 120°C for 10h, and finally the dried precipitated product was calcined in air at 600°C for 4h to obtain catalyst sample 1 # .
Embodiment 2
[0032] Weigh 9.7gNi(NO 3 ) 2 ·6H 2 O, 1.01g Ce(NO 3 ) 3 ·6H 2 O, 52.2gAl(NO 3 ) 3 9H 2 O was dissolved in 100mL deionized water and stirred to dissolve into a salt solution, and another 50g (NH 4 ) 2 CO 3 Solid solution 150mL deionized water to make alkaline solution, at 60°C, adopt co-current co-precipitation method, carry out co-precipitation reaction of the above two prepared solutions, control the pH value of the mother solution to 8 during the precipitation process, and continue to stir after the precipitation is completed After aging for 3 hours, the precipitated product was washed with deionized water, then dried at 120°C for 10 hours, and finally the dried precipitated product was calcined in air at 600°C for 4 hours to obtain catalyst sample 2 # .
Embodiment 3
[0034] Weigh 7.0gNi(NO 3 ) 2 ·6H 2 O, 0.49gLa(NO 3 ) 3 ·6H 2 O, 1.27gMg(NO 3 ) 2 ·6H 2 O, 57.4gAl(NO 3 ) 3 9H 2 O was dissolved in 100mL deionized water and stirred to dissolve into a salt solution, and another 50g (NH 4 ) 2 CO 3 Solid solution 150mL deionized water is made into alkaline solution. At room temperature, the two solutions prepared above are subjected to co-precipitation reaction by co-current co-precipitation method. During the precipitation process, the pH value of the mother solution is controlled to be 8. After the precipitation is completed, continue to stir and age 3h, then the precipitated product was washed with deionized water, and then dried at 120°C for 10h, and finally the dried precipitated product was roasted in air at 600°C for 4h to obtain catalyst sample 3 # .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com