Application of microbes in screening of drugs for treating eye diseases
A technology of microorganisms and microbial infection, which is applied in the field of diagnosis and treatment of ophthalmic diseases, and can solve the problems that eye diseases cannot be checked, and the detection method of PM2.5 eye diseases has not been disclosed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] The preparation of embodiment 1 culture medium model
[0090] The specific operation steps are: pour bacterial culture medium into a petri dish, purchased from China Guangzhou Huankai Microbiology Co., Ltd., the composition of the bacterial culture medium is as follows: each liter of double pure water contains 5g of peptone, 3g of beef extract, and 5g of sodium chloride , agar 15g and MnSO 4 5 mg. Sterilize the Petri dish containing the culture medium in an autoclave at 121°C for 30 min and cool down. Each flask was inoculated with approximately 1*10 7 A Bacillus megaterium was cultured in an incubator at 37°C for 24 hours to prepare a culture medium model.
Embodiment 2
[0091] Embodiment 2 culture medium model is used for drug screening
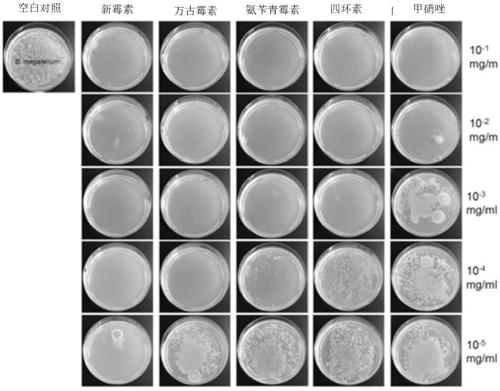
[0092] In order to test whether antibiotics can control the growth of Bacillus megaterium in vitro, we conducted an antibiotic sensitivity screening test in the culture medium model prepared in Example 1. The sensitivity of Bacillus megaterium to five major antimicrobial agents, ampicillin, vancomycin, neomycin, metronidazole and tetracycline, was examined using the minimum inhibitory concentration (MIC) method. Add different concentrations (10 -1 、10 -2 、10 -3 、10 -4 、10 -5 mg / mL) of ampicillin, vancomycin, neomycin, metronidazole, tetracycline, the above-mentioned antibacterial agents are all purchased from Sigma Corporation of the United States, and the experimental results are as follows: figure 1 As shown, by comparing with blank control and group inspection, it is found that Bacillus megaterium has the highest sensitivity to neomycin, followed by vancomycin, and metronidazole has the lowest effici...
Embodiment 3
[0093] Example 3 Preparation of aqueous humor / vitreous humor culture model and drug screening
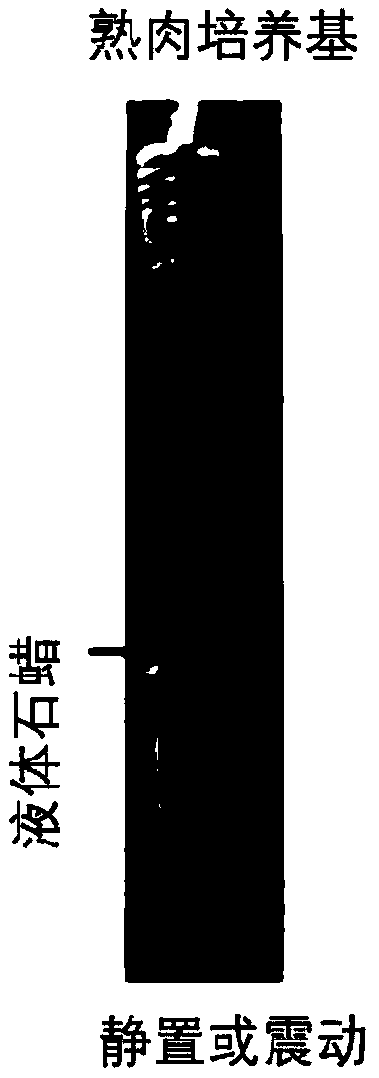
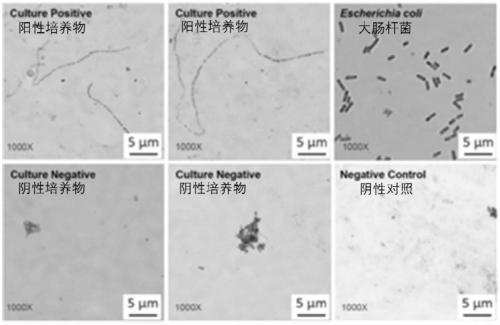
[0094] Cooked meat medium was purchased from Guangzhou Huankai Microbiology Co., Ltd., China, and its ingredients were: Lab-Lemco' powder 5g / L, peptone 30g / L, yeast extract 5g / L, NaH 2 PO 45g / L, glucose 3g / L, soluble starch 2g / L, no antibiotics. Add 6ml of cooked meat culture medium and sterilized dried beef grains into a 15ml glass tube, and add 1.5ml of liquid paraffin to seal it as a culture system. All tubes were then sterilized at 121°C for 30 minutes and cooled. Aqueous humor or vitreous humor (with and without drug) was injected into the glass tube above, and then cultured in a ZQTY-70F incubator at 37°C for 72 hours with shaking (200rpm) to prepare the aqueous humor / vitreous humor culture model. Cultures without aqueous humor or vitreous humor samples were used as negative controls, and E. coli cultures were used as positive controls. All cultures were then Gram-stained ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com