Synthesis method of propranolol hydrochloride
A technology of propranolol hydrochloride and a synthesis method, which is applied in the field of -1,2-propylene oxide, can solve the problems of unfavorable separation and purification, unfavorable industrialized production, and high raw material cost, and achieves easy industrialized production, low cost and simple operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
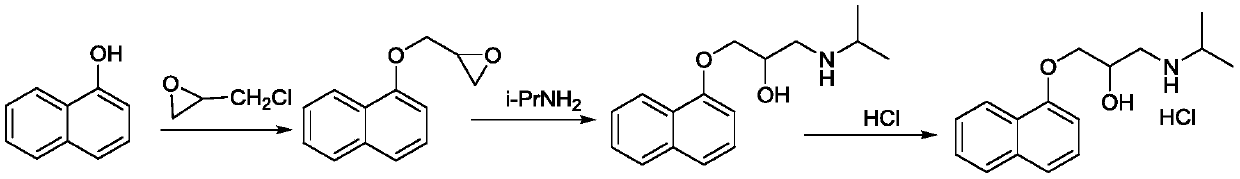
[0026] Example 1-1 Synthesis of 3-(1-naphthyloxy)-1,2-propylene oxide
[0027] Weigh methylnaphthol (144.1g, 1.0mol, 1.0eq), benzyltriethylammonium chloride (13.9g, 0.05mol, 0.05eq), epichlorohydrin (277.5g, 3.0mol, 3.0eq) and add Put it into a 1L four-neck flask, stir and raise the temperature to 50°C to dissolve and clarify, then add dropwise 30% NaOH aqueous solution (200g, 1.5mol, 1.5eq) for 1h, then control the temperature at 50°C for 6h, and monitor the disappearance of the raw material (V PE / EA= 5:1), stop the reaction, cool to room temperature, stand to separate the organic layer, wash once with water, and then concentrate the organic layer to dryness under reduced pressure at 50°C to obtain reddish-brown oil 3-(1-naphthyloxy)- 188.4 g of 1,2-propylene oxide, and the molar yield was 94.1%.
Embodiment 1-2
[0028] Example 1-2 Synthesis of 3-(1-naphthyloxy)-1,2-propylene oxide
[0029] Weigh methyl naphthol (216.2g, 1.5mol, 1.0eq), polyethylene glycol 6000 (18.0g, 0.03mol, 0.02eq), epichlorohydrin (347g, 3.75mol, 2.5eq) and join in 2L In an open flask, stir and heat up to 65°C to dissolve and clarify, then add dropwise 30% NaOH aqueous solution (320g, 2.4mol, 1.6eq) for 1h, then keep the temperature at 65°C for 4h, and TLC monitors that the raw material disappears (V PE / EA= 5:1), stop the reaction, cool to room temperature, stand to separate the organic layer, wash once with water, and then concentrate the organic layer to dryness under reduced pressure at 50°C to obtain reddish-brown oil 3-(1-naphthyloxy)- 286.5 g of 1,2-propylene oxide, and the molar yield was 95.4%.
Embodiment 2-1
[0030] Embodiment 2-1 propranolol is synthesized
[0031] Dissolve 3-(1-naphthyloxy)-1,2-epoxypropane (160g, 0.8mol, 1.0eq), isopropylamine (108g, 1.6mol, 2.0eq) in 300mL of toluene, and then add N , N-diisopropylethylamine (25.9g, 0.2mol, 0.25eq), added dropwise for 30min, after the dropwise completion, the temperature was raised to 45°C, and the reaction was kept for 4h, and the raw material disappeared under TLC monitoring (V CHCl3 / MeOH =20:1), stop the reaction, cool to 5°C, precipitate solid, filter and dry to obtain 189.4g of propranolol crude product, the yield is 91.3%, and the HPLC purity is 99.1%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com