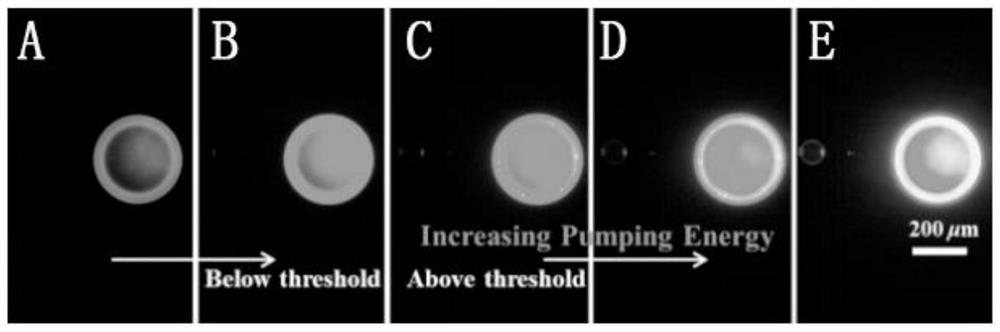
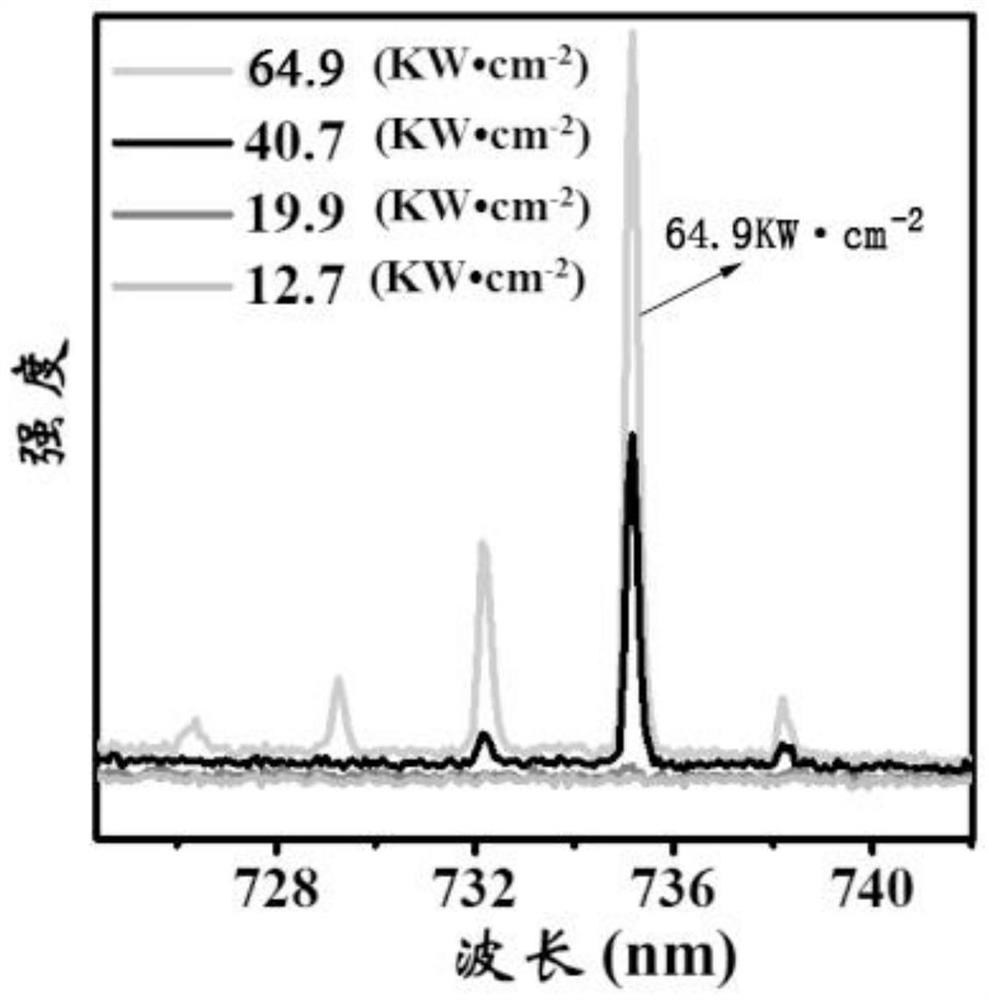
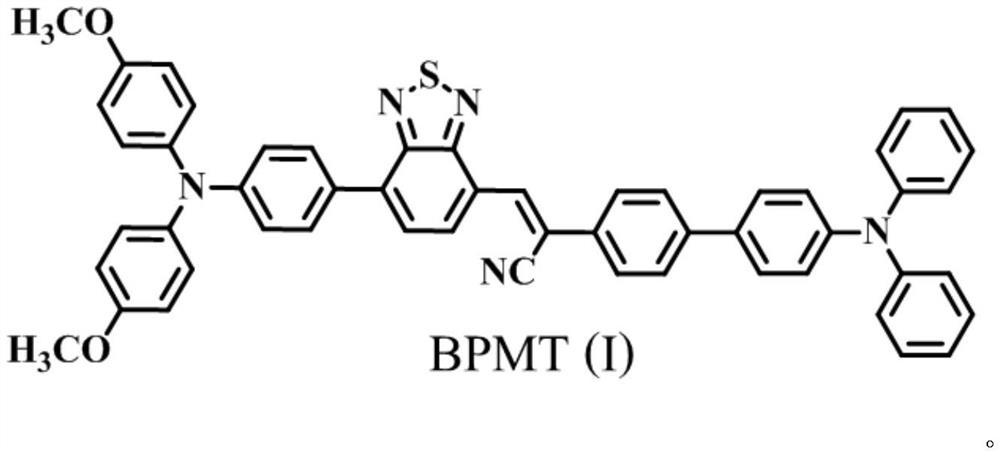
A kind of efficient organic near-infrared fluorescent material and its preparation and application
A fluorescent material and near-infrared technology, applied in the fields of luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problem of low photoluminescence efficiency, and achieve the effect of strong penetrating ability, avoiding autofluorescence and low excitation energy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] The preparation method of the above-mentioned high-efficiency organic near-infrared fluorescent material includes:
[0035] (S1) Synthesis of Intermediate Benzothiadiazole Derivatives (Ⅱ)
[0036] The synthetic route of this benzothiadiazole derivative (II) is as follows:
[0037]
[0038] Weigh (4-(2'-(4"-methoxyphenyl)amino)phenyl)boronic acid, 7-bromo-4-formylbenzothiadiazole, tetrakis(triphenylphosphine)palladium dissolved in In the mixed solution of toluene / tetrahydrofuran, add an inorganic strong alkali solution (K 2 CO 3 solution). Among them, (4-(2'-(4″-methoxyphenyl)amino)phenyl)boronic acid: 7-bromo-4-formylbenzothiadiazole: tetrakis(triphenylphosphine) palladium: inorganic Strong base (K 2 CO 3 ) molar ratio is 2:2~3:0.1~0.3:3~5; the volume ratio of toluene and tetrahydrofuran is 1~2:1~2. Under the usage ratio of the raw materials and the solvent and the solvent ratio, the degree of completion of the reaction is good and the yield is high. Under ni...
Embodiment 1
[0047] A method for preparing an efficient organic near-infrared fluorescent material, comprising:
[0048] (S1) Synthesis of intermediate benzothiadiazole derivatives (II):
[0049] Weigh (4-(2'-(4″-methoxyphenyl)amino)phenyl)boronic acid 2mmol (0.698g), 7-bromo-4-formylbenzothiadiazole 2mmol (0.486g), Four (triphenylphosphine) palladium 0.1mmol (0.116g), then add 3mmol (0.415g) K 2 CO 3 Dissolve in a mixed solution of 30mL toluene and 30mL tetrahydrofuran. Under nitrogen conditions, the temperature was raised to 90° C. for reflux reaction for 10 h.
[0050] After the reaction solution was cooled, extraction was performed, and the organic phases were combined, and dried by adding anhydrous magnesium sulfate. The residue obtained by filtration and concentration under reduced pressure was separated by silica gel column chromatography, the eluent was (petroleum ether / dichloromethane=3:1), and the dark red intermediate benzothiadiazole derivative was obtained after the solven...
Embodiment 2
[0060] (S1) Synthesis of intermediate benzothiadiazole derivatives (II):
[0061] Weigh (4-(2'-(4″-methoxyphenyl)amino)phenyl)boronic acid 2mmol (0.698g), 7-bromo-4-formylbenzothiadiazole 3mmol (0.729g), Four (triphenylphosphine) palladium 0.1mmol (0.116g), then add 3mmol (0.415g) K 2 CO 3 Dissolve in a mixed solution of 30mL toluene and 30mL tetrahydrofuran. Under nitrogen conditions, the temperature was raised to 90° C. for reflux reaction for 10 h.
[0062] After the reaction solution was cooled, extraction was performed, and the organic phases were combined, and dried by adding anhydrous magnesium sulfate. The residue obtained by filtration and concentration under reduced pressure was separated by silica gel column chromatography, the eluent was (petroleum ether / dichloromethane=3:1), and the dark red intermediate benzothiadiazole derivative was obtained after the solvent was rotary evaporated under reduced pressure. The compound (Ⅱ) was 0.832g, and the total yield was ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com