Preparation method of borate with polymerizable double bonds
A boric acid ester and boronic acid technology, applied in the field of organic boronate compounds, can solve the problems of insufficient application performance of coupling agents, reduce nucleophilic attack rate constants and other problems, and achieve the effect of good compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
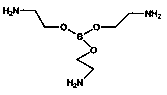
[0028] 1. Preparation of borate esters with polymerizable double bonds
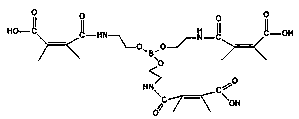
[0029] In the first step, add 0.1 mol of boric acid and 0.3 mol of triethanolamine to a three-necked flask equipped with a stirrer and a water separator, then add 100 mL of toluene, stir and heat at an oil bath temperature of 130 ° C, and use a water separator to separate water, react until anhydrous is produced, and after cooling, there is a light yellow solid insoluble in toluene, and the toluene is filtered to obtain a solid, washed with a small amount of dimethylformamide for 2 to 3 times, and then washed with absolute ethanol for 2 to 3 times, and then Recrystallize in absolute ethanol, vacuum-dry the crystallized product at 60°C, and finally obtain triethanolamine borate with smooth surface and granular form as shown in structural formula (I). In the second step, 0.1 mol of triethanolamine borate synthesized in the first step was dissolved in N,N-dimethylformamide, and 0.3 mol of butadienedioic acid...
Embodiment 2
[0033] The preparation process is the same as in Example 1, wherein toluene is replaced by chloroform. The yield was 84.3%.
Embodiment 3
[0035] The preparation process is the same as in Example 1, the temperature of the oil bath in the first step is 120°C, and the heating temperature in the second step is 80°C. The yield was 83.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com