Ray chondroitin sulfate and extraction method thereof
A technology of chondroitin sulfate and chondroitin, which is applied in the direction of pharmaceutical formulations, organic active ingredients, anti-toxic agents, etc., can solve the problems of incomplete hydrolysis of collagen and mucin, pollution of the environment, etc., and achieve strong ability to scavenge free radicals, strong The effect of antioxidant capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Comparison of the pros and cons of different skate chondroitin sulfate extraction methods in embodiment 1
[0042] Concentrated alkali-concentrated salt method, dilute alkali-enzymatic hydrolysis method and ultrasonic-assisted alkali-salt-enzymatic hydrolysis method were used to extract cartilage of ray cartilage, and the extraction rate of chondroitin sulfate, glucuronic acid content and appearance quality were used as indicators to compare each Pros and cons of extraction methods.
[0043]1. Extraction process of ray chondroitin sulfate by concentrated alkali-concentrated salt method
[0044] Ray cartilage powder → 10% NaOH, 20% NaCl → 40°C extraction → adjust the pH of the filtrate to 7-8 → ethanol precipitation → centrifuge to take the precipitate → freeze-drying → CS;
[0045] 2. Extraction process of ray chondroitin sulfate by dilute alkali-enzymatic hydrolysis
[0046] Ray cartilage powder → 2% NaOH, 2% NaCl → 40 ℃ alkali salt extraction → adjust pH to 9 → 50 ℃...
Embodiment 2
[0053] Example 2 Ultrasonic assisted alkali salt-enzymolysis method to extract chondroitin sulfate single factor process parameter test
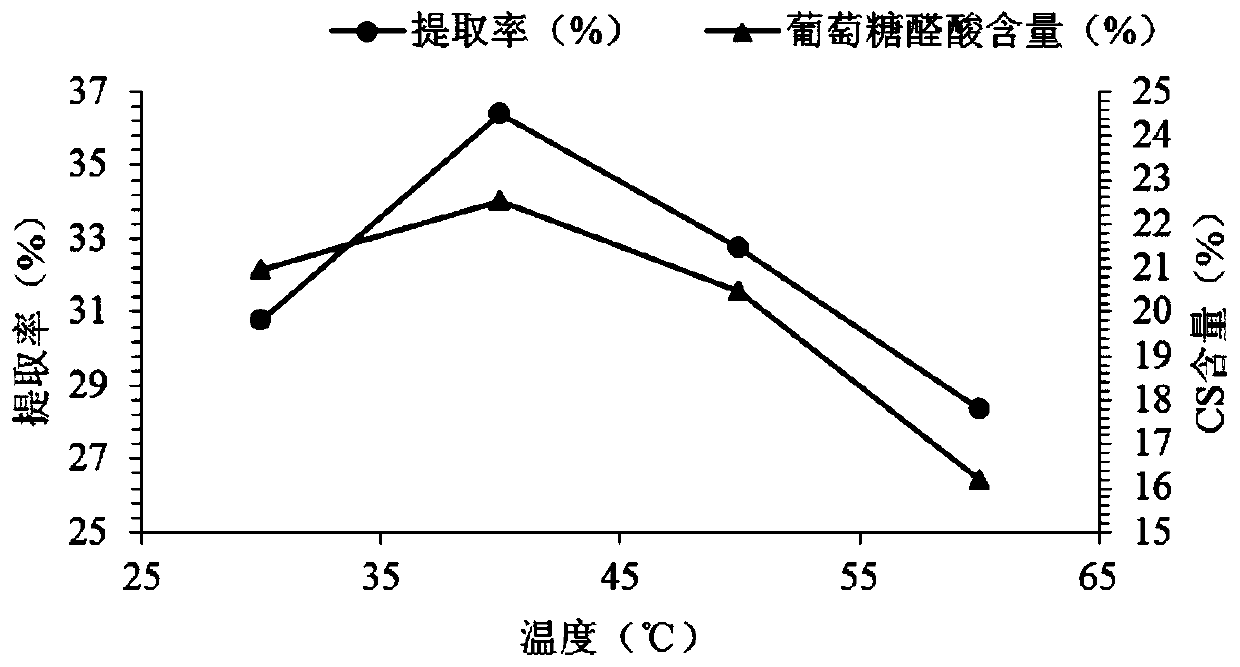
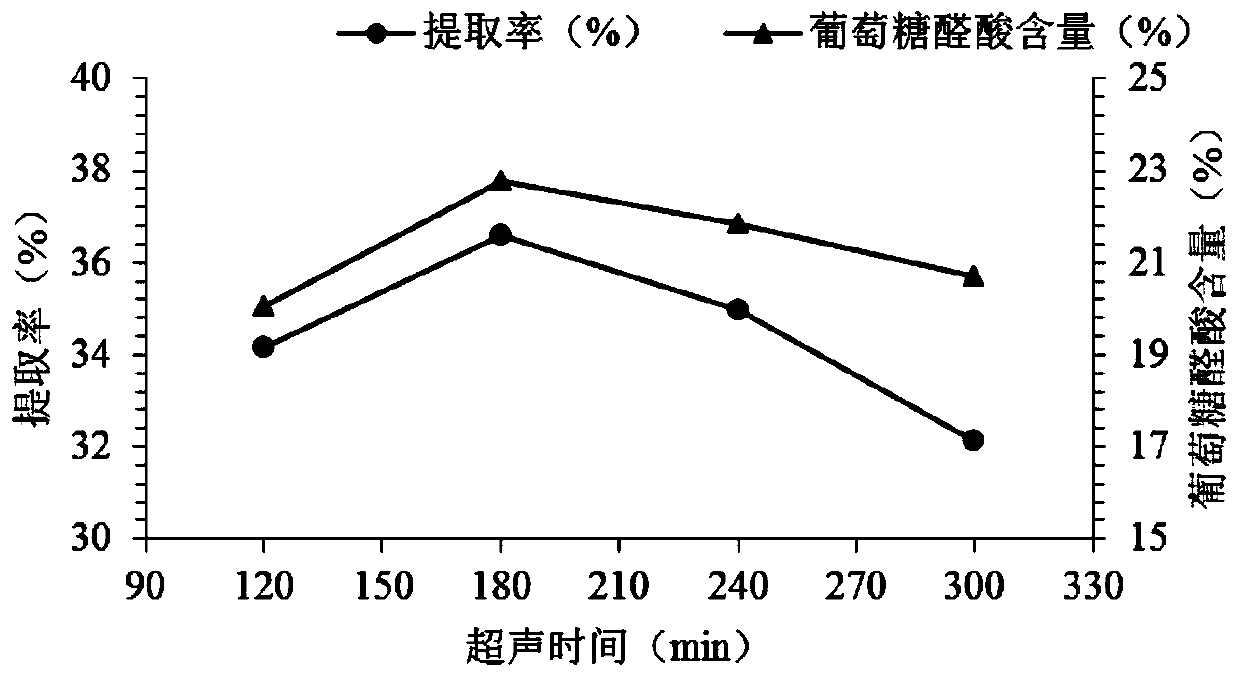
[0054] Taking the extraction rate of CS and the content of glucuronic acid as the evaluation index, the six effects of lye concentration, extraction temperature, ultrasonic extraction time, alkaline protease addition, enzymolysis temperature and enzymolysis time on the extraction of ray chondroitin sulfate were respectively evaluated. The key process parameters of the effect are subjected to a single factor test, and the factor levels of the single factor test are shown in Table 2.
[0055] Table 2 Factor level table of single factor experiment
[0056]
[0057] The formula for calculating the extraction rate of chondroitin sulfate is In the formula: W—chondroitin sulfate extraction rate, %; m—freeze-dried chondroitin sulfate product quality, g; 5—mass of skate cartilage powder, g.
Embodiment 21
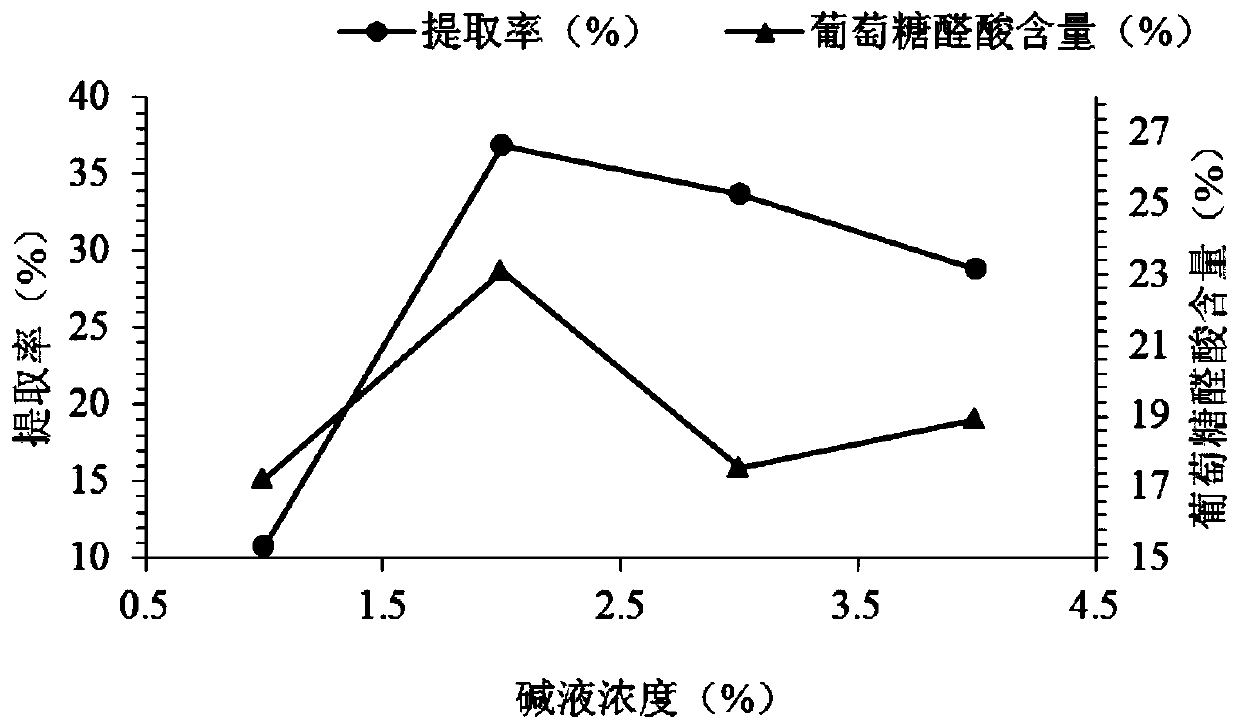
[0058] Embodiment 2.1 lye concentration single factor test
[0059] Weigh 5g of ray cartilage powder into a beaker for each group of tests, add 2%NaCl-1%NaOH, 2%NaCl-2% respectively according to the design of Table 2-3 with a solid-liquid ratio of 1:6g / mL Add NaOH, 2% NaCl-3% NaOH, 2% NaCl-4% NaOH aqueous solution, stir while adding to make it fully mixed, and ultrasonically extract at 40°C for 180min. After taking it out, filter it with double-layer gauze, collect the filtrate, adjust the pH to 11 after cooling, add 0.5% (mass volume ratio) alkaline protease, and shake at 45°C for 12h. After taking out, inactivate the enzyme at 90°C for 10min, centrifuge at 5000r / min for 10min, take the supernatant, precipitate with ethanol with a final concentration of 75%, let stand at 4°C for 24h, collect the precipitate by centrifugation and freeze-dry to obtain CS polysaccharide. The CS was weighed in time, stored at 4°C under dry conditions, and its content was to be determined.
[00...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com