Method for regulating and controlling preparation of nano calcium carbonate by using crystal form control agent
A crystal form control agent, nano-calcium carbonate technology, applied in the direction of calcium carbonate/strontium/barium, nanotechnology, chemical instruments and methods, etc., can solve the problem of difficult to obtain ultra-fine nano-calcium carbonate products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
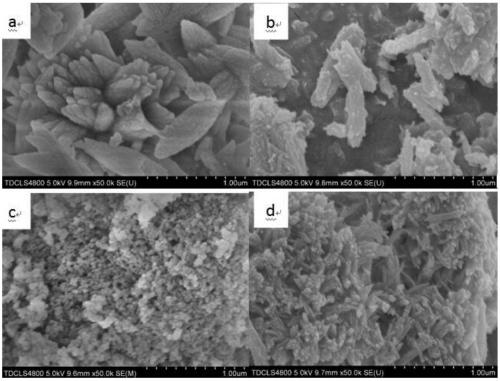
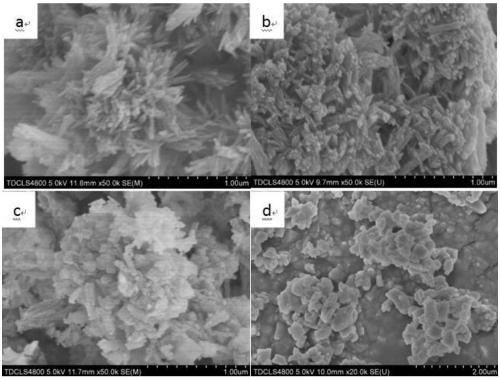
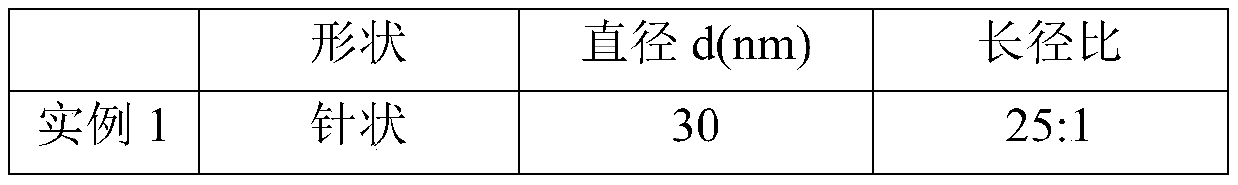
[0019] Put the limestone in a muffle furnace for calcination at 1050°C, set the heating rate to 10°C / min, and hold the time for 2 hours to obtain quicklime powder; grind the quicklime powder and pass through a 200-mesh sieve, Add 1:20 to 80°C deionized water and stir for 2 hours to obtain a mass fraction of 17.8% Ca(OH) 2 Emulsion; resulting Ca(OH) 2 Dilute the emulsion with water to obtain Ca(OH) with a mass fraction of 5% 2 Emulsion; for Ca(OH) 2 The emulsion is aged to obtain a suspension of slaked lime, and the aging time is 48 hours; the suspension of slaked lime is added with EDTA-2Na accounting for 5% of the mass of calcium hydroxide, placed in a low-temperature water tank, and the temperature is controlled by ice water to be 3 degrees Celsius. Stir at a speed of 400rpm and feed in CO with a flow rate of 2L / h per gram of calcium hydroxide 2 Gas, real-time monitoring of the pH value of the emulsion, when the pH value is 7, stop feeding CO 2 , and then stirred for 2 h...
Embodiment 2
[0021] The specific process is as in Example 1, the difference is: in the calcination process, the calcination condition is 950 ° C for 4 hours to obtain calcium oxide powder; when the quicklime is digested, the solid-to-liquid molar ratio of the sieved powder and water becomes 1 :5, the stirring time is reduced to 0.5h, and the Ca(OH) that the mass fraction is obtained is 50% 2 Emulsion; dilute with water, after 30 hours of aging to obtain a suspension of slaked lime with a mass fraction of 20%; when slaked lime is carbonized, the crystal form control agent adopts sodium citrate accounting for 2% of the mass of calcium hydroxide, and the stirring speed is 100rpm, CO 2 The flow rate of the gas is controlled at 0.5L / h per gram of calcium hydroxide; when drying, it is dried at 60°C for 2 hours; finally, rod-shaped ultrafine nano-calcium carbonate is obtained.
Embodiment 3
[0023] The specific process is as in Example 1, the difference is that: during the calcination process, the calcination condition is 1050 ° C for 2 hours to obtain calcium oxide powder; when the quicklime is digested, the solid-to-liquid molar ratio of the sieved powder and water becomes 1 :10, the stirring time is reduced to 1.5h, and the Ca(OH) that obtains mass fraction is 31.3% 2 Emulsion; dilute with water, after 24 hours of aging to obtain a suspension of slaked lime with a mass fraction of 10%; when slaked lime is carbonized, the crystal form control agent adopts zinc sulfate accounting for 3% of the mass of calcium hydroxide, and the stirring speed is 200rpm, CO 2 The flow rate of the gas is controlled at 1.5 L / h per gram of calcium hydroxide; when drying, it is dried at 60°C for 2 hours; finally, blocky cubic ultrafine nano-calcium carbonate is obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com