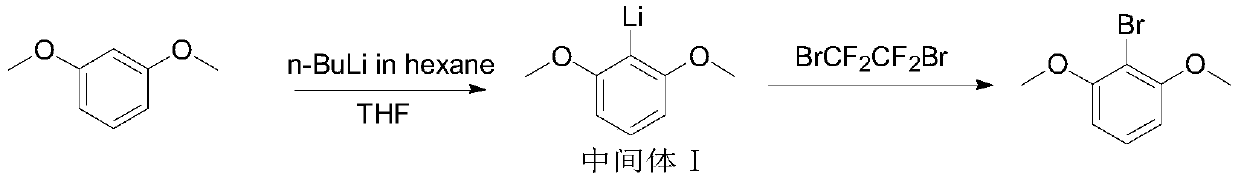
Preparation method of 2-bromo-1, 3-dimethoxybenzene
A technology of dimethoxybenzene and m-phenylenedimethoxybenzene, which is applied in the field of preparation of high-purity 2-bromo-1,3-dimethoxybenzene, can solve the problems of unsuitable for green industrial production, poor reaction selectivity, Purification is difficult and difficult to achieve the effect of convenient operation, less side reactions and high reaction conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] This example provides a preparation method for synthesizing high-purity 2-bromo-1,3-dimethoxybenzene, the steps of which are as follows:
[0025] (1) Prepare a 10L glass reaction kettle, first add 4L tetrahydrofuran and m-xylylene dimethyl ether (500g, 3.62mol) in sequence, stir to dissolve, and form a colorless transparent solution; cool to 5-10°C in an ice-water bath, slowly add 1.5L dropwise n-BuLi in hexane (2.3mol / L) (3.45mol), drop slowly within 1.5h, gradually become cloudy after adding, with exothermic, return to room temperature after dropwise addition, continue at 20~25℃ , after reacting for 1 hour, the intermediate I was obtained, sampled and detected, and the reaction status of the lithium salt of the intermediate I was confirmed by NMR;
[0026] (2) After confirming that the reaction is complete, first lower the temperature of the reaction system intermediate I, use a dry ice-acetone bath, cool to -65°C, and slowly add 1,2-dibromotetrafluoroethane (408ml, 3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com