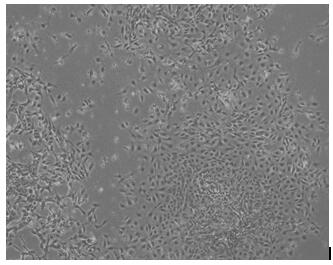
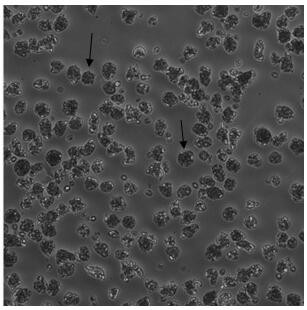
A method for directed differentiation of autologous immune cells into islet cells
A directional differentiation, islet cell technology, applied in animal cells, pancreatic cells, vertebrate cells, etc., can solve the problems of long cell culture time, low transformation rate, low insulin secretion level of islet cells, etc., to reduce immune rejection. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Immune cells obtained from peripheral blood of patients were dedifferentiated into induced pluripotent stem cells in vitro
[0043] The method in this example refers to the method for inducing induced pluripotent stem cells in CN108642014 A patent. Dilute 50 mL of peripheral blood with normal saline at a ratio of 1:1. Carefully add the diluted blood to the same volume of lymphocyte separation medium to form obvious layers, and centrifuge horizontally at 900g for 25min at room temperature. At this time, four layers are formed in the centrifuge tube from top to bottom, and the buffy coat layer of the second layer is taken out as much as possible. Add 2 times of normal saline, wash the cells twice, discard the supernatant after centrifugation, and collect immune cells.
[0044] After the immune cells were cultured overnight in RPMI medium, the prepared lentivirus containing four genes of OCT4, SOX2, KLF4, and C-MYC was used to infect the immune cells, cultured, s...
Embodiment 2
[0045] Example 2 Directed differentiation of induced pluripotent stem cells to generate islet cells
[0046] The directed differentiation of induced pluripotent stem cells induces the generation of endoderm cells, the steps are as follows: separate the induced pluripotent stem cells with a separation medium, blow off the cell mass with a pipette, and inoculate in a culture medium coated with 0.1% fibronectin. When the cells plated to about 60%, they were cultured in S1 medium, and 14ug / ml of CHIR99021 compound was added on the first day to induce the cells. The next day, fresh S1 medium was replaced (without adding CHIR99021 compound), and cultured for 2 days to obtain definitive endoderm cells. The cells were collected, and the expressions of definitive endoderm marker genes SOX17 and FOXA2 were identified by RT-qPCR.
[0047] The endoderm cells continue to differentiate and induce protogut tubes, the medium is replaced with S2 medium every other day, and cultured for 3 days...
Embodiment 3
[0059] Example 3 RT-qPCR method to detect the expression of cell-specific markers at each stage
[0060] Collect the cells on the last day of S1, S4, and S6 stages, use conventional methods to extract total RNA, synthesize cDNA, and q-PCR to amplify the target gene. The specific markers detected include SOX17, FOXA2, PDX1, NKX6-1, INS , GCG, SST, the results are attached Figure 4-6 . The primer sequence was synthesized by Beijing Biomed Biotechnology Co., Ltd. The primer sequence and annealing temperature of the gene are shown in the table below.
[0061]
[0062] Depend on Figure 4-6 It can be seen that the endoderm-specific marker SOX17 is strongly expressed in the definitive endoderm stage, FOXA2 is also highly expressed in the definitive endoderm stage, and the transcription factor PDX1 related to pancreatic development and the transcription factor NKX6-1 related to pancreatic endoderm are in the original Expression is elevated when enterocytes are induced to the p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com